Label: ZULRESSO- brexanolone injection, solution
- NDC Code(s): 72152-547-20
- Packager: Sage Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated July 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZULRESSO safely and effectively. See full prescribing information for ZULRESSO. ZULRESSO® (brexanolone) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: EXCESSIVE SEDATION AND SUDDEN LOSS OF CONSCIOUSNESS
Patients treated with ZULRESSO are at risk of excessive sedation or sudden loss of consciousness during administration [see Warnings and Precautions (5.1)].
Because of the risk of serious harm, patients must be monitored for excessive sedation and sudden loss of consciousness and have continuous pulse oximetry monitoring. Patients must be accompanied during interactions with their child(ren) [see Warnings and Precautions (5.1)].
Because of these risks, ZULRESSO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ZULRESSO REMS [see Warnings and Precautions (5.2)].
Close -
1 INDICATIONS AND USAGEZULRESSO is indicated for the treatment of postpartum depression (PPD) in patients 15 years and older [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION2.1 Important Considerations Prior to Initiating and During Therapy - A healthcare provider must be available on site to continuously monitor the patient, and intervene as necessary, for the ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 100 mg/20 mL (5 mg/mL) clear, colorless solution in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Excessive Sedation and Sudden Loss of Consciousness - In clinical studies in adults, ZULRESSO caused sedation and somnolence that required dose interruption or reduction in some patients ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Excessive Sedation and Sudden Loss of Consciousness [see Boxed Warning, Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants - Concomitant use of ZULRESSO with CNS depressants (e.g., opioids, benzodiazepines) may increase the likelihood or severity of adverse reactions related to sedation [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - ZULRESSO contains brexanolone, a Schedule IV controlled substance. 9.2 Abuse - In a human abuse potential study, 90 mcg/kg, 180 mcg/kg (two times the maximum ...
-
10 OVERDOSAGEHuman Experience - There is limited clinical trial experience regarding human overdosage with ZULRESSO. In premarketing clinical studies, two cases of accidental overdosage due to infusion pump ...
-
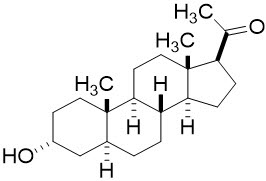
11 DESCRIPTIONZULRESSO contains brexanolone, a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator, that is chemically identical to endogenous allopregnanolone. The molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of brexanolone in the treatment of PPD is not fully understood, but is thought to be related to its positive allosteric modulation of GABAA ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies of brexanolone have not been performed. Mutagenesis - Brexanolone was not genotoxic when ...
-
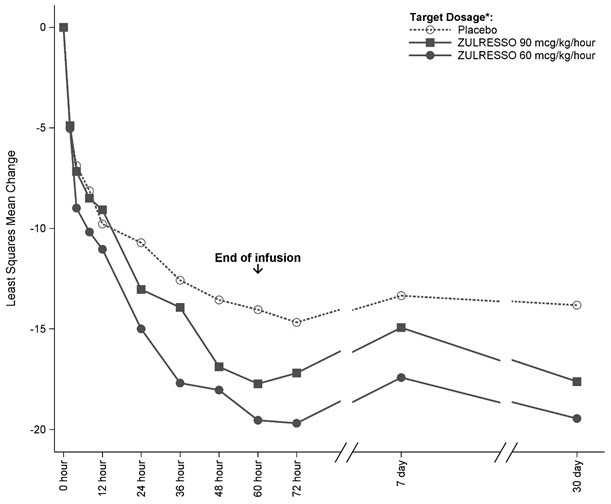
14 CLINICAL STUDIESThe efficacy of ZULRESSO in the treatment of postpartum depression (PPD) was demonstrated in two multicenter, randomized, double-blind, placebo-controlled studies (referred to as Studies 1 and 2 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ZULRESSO injection is supplied as 100 mg brexanolone in 20 mL (5 mg/mL) single-dose vials containing a sterile, preservative-free, clear, colorless solution. NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Excessive Sedation and Sudden Loss of Consciousness - Patients may experience loss of consciousness or altered ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. v3.0 Revised: 6/2022 - MEDICATION GUIDE - ZULRESSO® (zul reh' soe ...
-
PRINCIPAL DISPLAY PANEL - 20 mL Vial LabelNDC 72152-547-20 - Rx ONLY - 20 mL - Zulresso® (brexanolone) injection - 100 mg/20 mL - (5 mg/mL) CIV - FOR INTRAVENOUS INFUSION - AFTER DILUTION.
-
PRINCIPAL DISPLAY PANEL - 20 mL Vial CartonRx ONLY - NDC 72152-547-20 - 20 mL - Zulresso® (brexanolone) injection - 100 mg/20 mL - (5 mg/mL) CIV - FOR INTRAVENOUS INFUSION - AFTER DILUTION - Dispense the accompanying - Medication Guide to each patient.
-
INGREDIENTS AND APPEARANCEProduct Information