Label: ZTLIDO- lidocaine patch
- NDC Code(s): 69557-111-01, 69557-111-03, 69557-111-30
- Packager: Scilex Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZTLIDO - ®safely and effectively. See full prescribing information for ZTLIDO - ®. ZTLIDO - ®(lidocaine topical system ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZTLIDO is indicated for relief of pain associated with post-herpetic neuralgia (PHN) in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Instructions - Because of the difference in bioavailability of ZTLIDO compared to Lidoderm (lidocaine patch 5%), a different dosage strength is required ...
-
3 DOSAGE FORMS AND STRENGTHSTopical system: 1.8% packaged in an individual envelope.
-
4 CONTRAINDICATIONSZTLIDO is contraindicated in patients with a known history of sensitivity to local anesthetics of the amide type, or to any other component of the product.
-
5 WARNINGS AND PRECAUTIONS5.1 Accidental Exposure - A used ZTLIDO topical system contains residual lidocaine after use. The potential exists for a small child or a pet to suffer serious adverse effects from chewing or ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: Excessive Dosing/Overexposure to Lidocaine - [see - Warnings and Precautions (5.2) ...
-
7 DRUG INTERACTIONS7.1 Drugs That May Cause Methemoglobinemia When Used with ZTLIDO - Patients who are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited human data with lidocaine in pregnant woman are not sufficient to inform drug-associated risk for major birth defects and miscarriage. The use of ...
-
10 OVERDOSAGELidocaine overdose from cutaneous absorption is rare, but could occur. If there is any suspicion of lidocaine overdose, check drug blood concentration. The management of overdose includes close ...
-
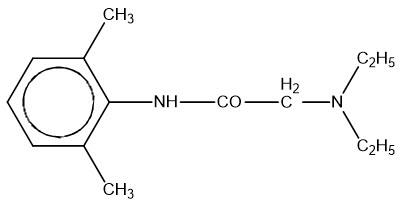
11 DESCRIPTIONZTLIDO (lidocaine topical system) 1.8% is a single-layer, drug-in-adhesive topical delivery system comprised of an adhesive material containing 36 mg lidocaine, which is applied to a pliable ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lidocaine is an amide local anesthetic. Lidocaine blocks sodium ion channels required for the initiation and conduction of neuronal impulses. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals specifically designed to evaluate the carcinogenic potential of lidocaine or ZTLIDO ...
-
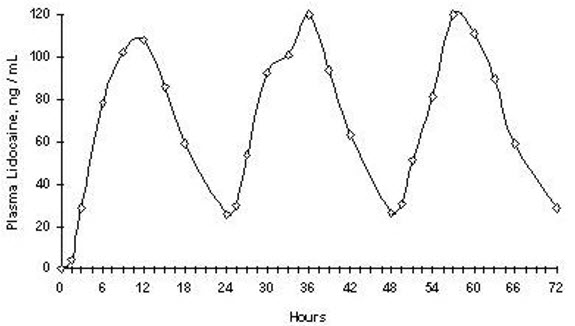
14 CLINICAL STUDIESSingle-dose treatment with lidocaine patch (currently preferred dosage form term for a patch is topical system) was compared to treatment with vehicle patch (without lidocaine), and to no ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZTLIDO (lidocaine topical system) 1.8% is available as the following: Carton of 30 topical systems, packaged into individual child-resistant envelopes. NDC 69557-111-30 - Store at 20° to 25°C ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Accidental Exposure and Disposal - Advise patients to store ZTLIDO out of the reach ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Scilex Pharmaceuticals Inc. Palo Alto, CA 94303 - USA - ZTLIDO - ®is a trademark owned by Scilex Pharmaceuticals Inc. Patented. See ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Issued: 4/2021 PATIENT INFORMATION - ZTLIDO - ®(ZEE-TEE-LIE-DOH ...
-
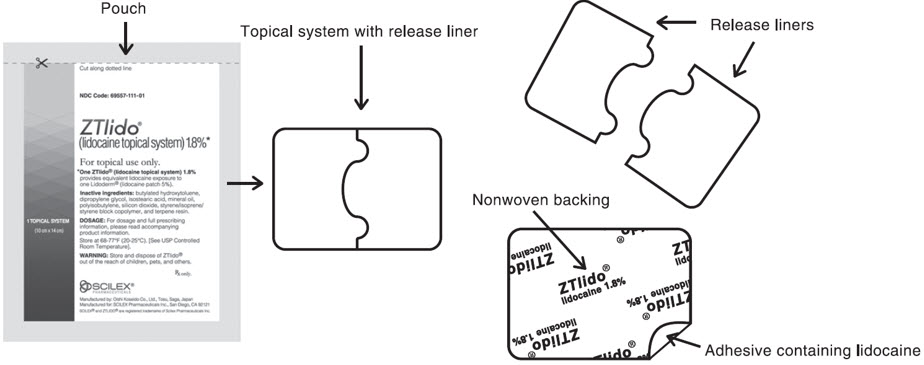
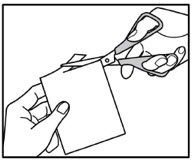
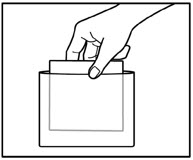
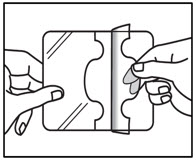
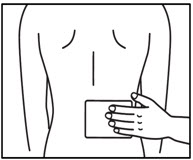
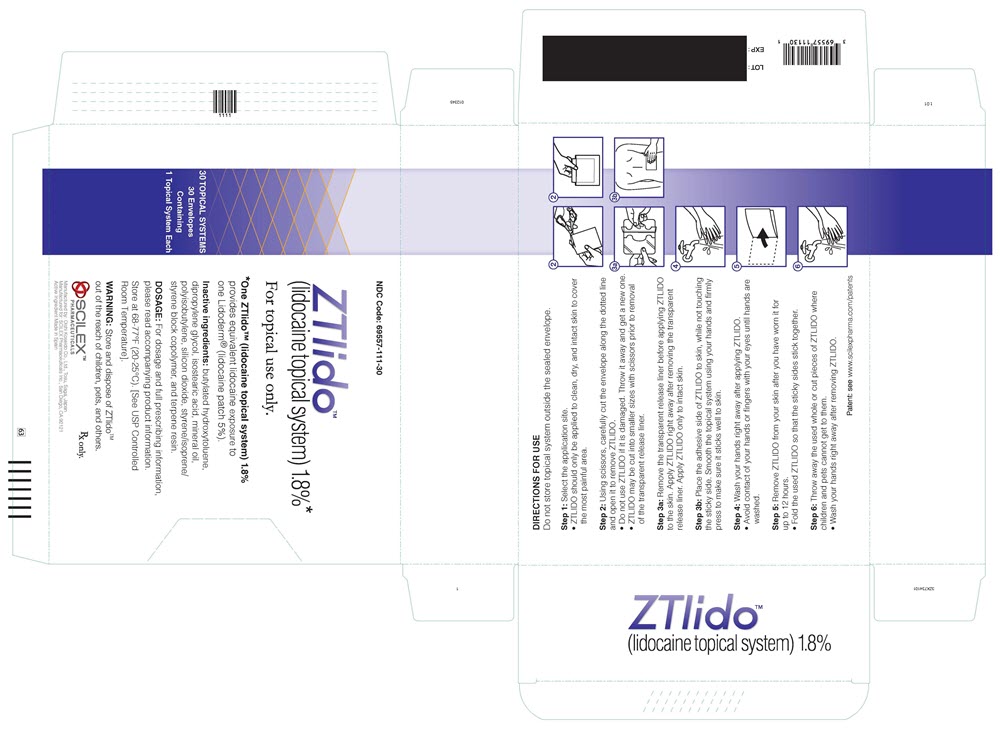
INSTRUCTIONS FOR USEInstructions for Use - ZTLIDO - ®(ZEE-TEE-LIE-DOH) (lidocaine topical system) Read this Instructions for Use before you start using ZTLIDO and each time you get a refill. There ...
-
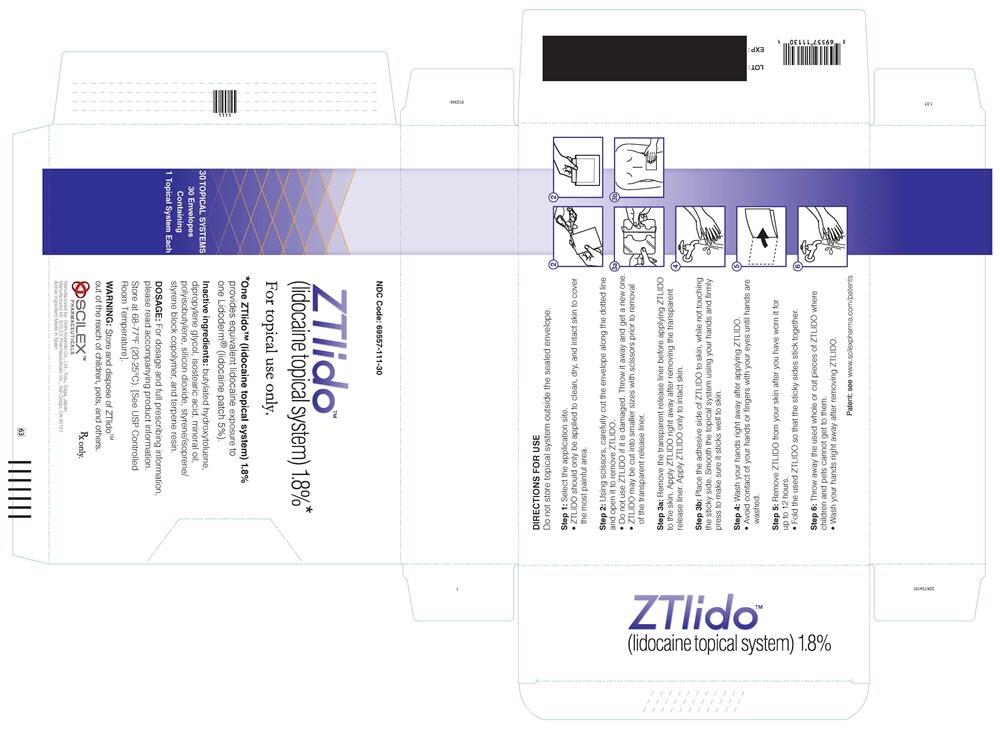
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - lidocaine patch 1.8% CARTON LABEL - NDC 69557-111-30 - ZTlido™ (lidocaine topical system) 1.8%* For topical use only. *One ZTlido™ (lidocaine topical system ...
-
INGREDIENTS AND APPEARANCEProduct Information