Label: ZORCAINE- articaine hydrochloride and epinephrine injection, solution
- NDC Code(s): 0362-0830-05
- Packager: Septodont, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Zorcaine® safely and effectively. See full prescribing information for Zorcaine®. Zorcaine® (articaine hydrochloride and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZorcaine® is indicated for local, infiltrative, or conductive anesthesia in both simple and complex dental procedures in adults and pediatric patients 4 years of age or older.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage Information - Table 1 summarizes the recommended dosages of ZORCAINE administered by intraoral submucosal infiltration or nerve block for various types of anesthetic dental ...
-
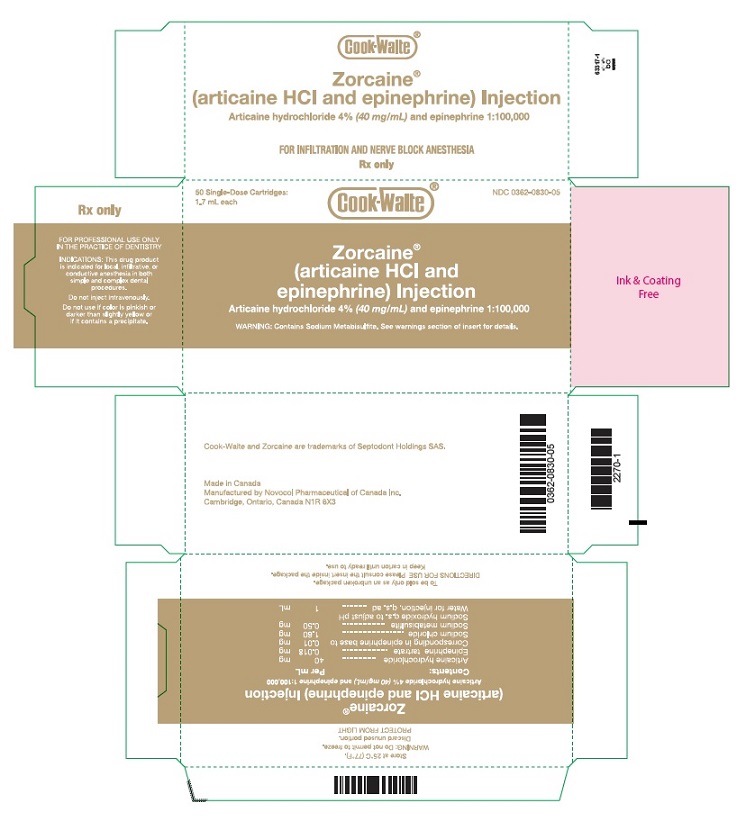
3 DOSAGE FORMS AND STRENGTHSInjection (clear, colorless solution), provided in: Glass cartridges (single-dose) containing (less than a full cartridge or more than one cartridge may be used for an individual patient): ...
-
4 CONTRAINDICATIONSZORCAINE is contraindicated in patients who are hypersensitive to products containing sulfites. Products containing sulfites may cause allergic-type reactions including anaphylactic symptoms and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Accidental Intravascular Injection - Accidental intravascular injection of ZORCAINE may be associated with convulsions, followed by central nervous system or cardiorespiratory depression and ...
-
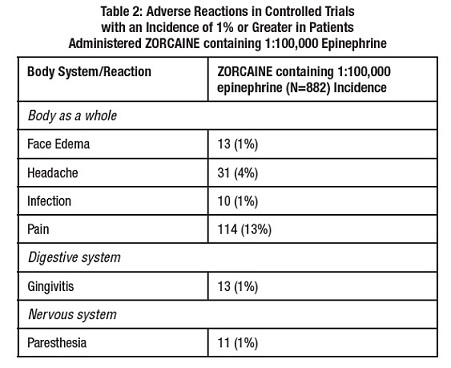
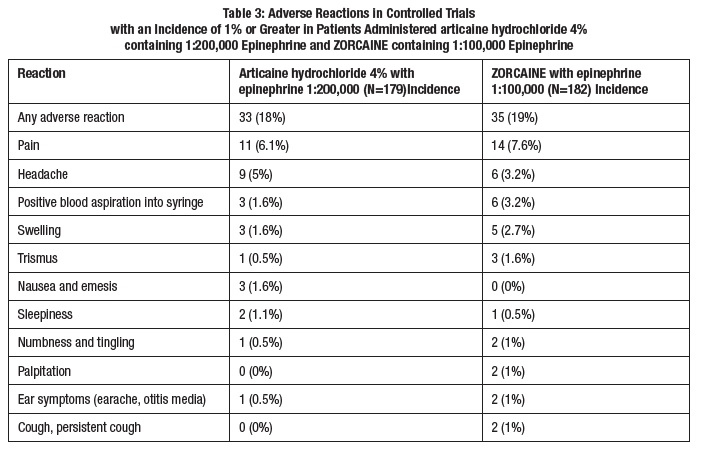
6 ADVERSE REACTIONSReactions to articaine are characteristic of those associated with other amide-type local anesthetics. Adverse reactions to this group of drugs may also result from excessive plasma levels (which ...
-
7 DRUG INTERACTIONSThe administration of local anesthetic solutions containing epinephrine to patients receiving monoamine oxidase inhibitors, nonselective beta-adrenergic antagonists, or tricyclic antidepressants ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects - Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women with ZORCAINE. Articaine hydrochloride and epinephrine ...
-
10 OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local ...
-
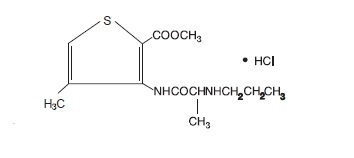
11 DESCRIPTIONZORCAINE, for intraoral submucosal infiltration use, is a sterile, aqueous solution that contains articaine HCl 4% (40 mg/mL) and epinephrine bitartrate in an epinephrine 1:200,000 or epinephrine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Articaine HCl is an amide local anesthetic. Local anesthetics block the generation and conduction of nerve impulses, presumably by increasing the threshold for ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies to evaluate the carcinogenic potential of articaine HCl in animals have not been conducted. Five standard mutagenicity tests ...
-
14 CLINICAL STUDIESThree randomized, double-blind, active-controlled studies were designed to evaluate effectiveness of ZORCAINE containing epinephrine 1:100,000 as a dental anesthetic. Patients ranging in age from ...
-
15 REFERENCESKaplan, EL, editor. Cardiovascular disease in dental practice. Dallas; American Heart Association; 1986.
-
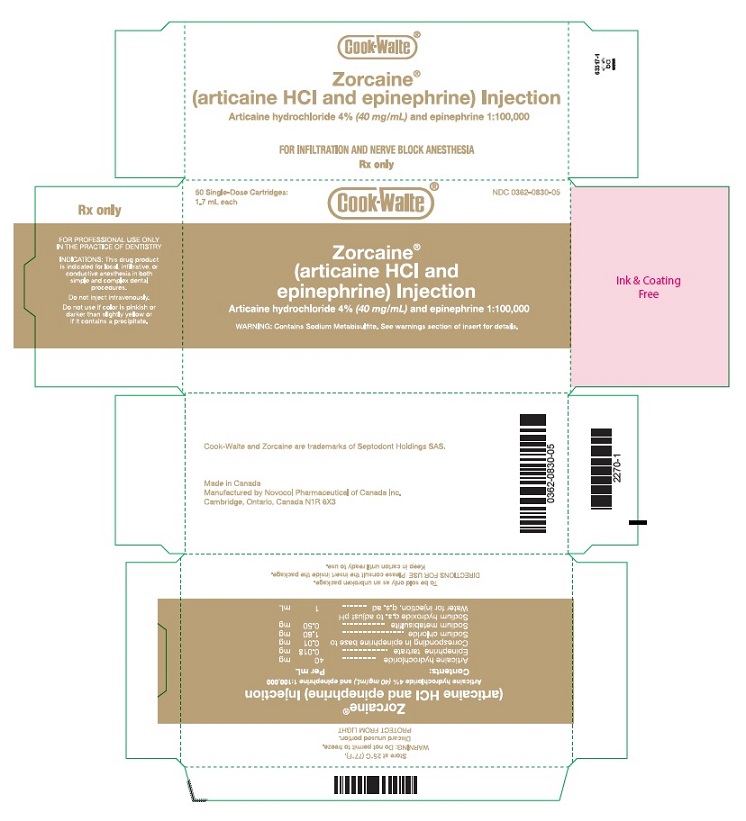
16 HOW SUPPLIED/STORAGE AND HANDLINGZORCAINE Injection is a clear, colorless solution available in 1.7 mL single-dose glass cartridges, packaged in boxes of 50 cartridges in the following two strengths (less than a full cartridge or ...
-
17 PATIENT COUNSELING INFORMATIONLoss of Sensation and Muscle Function: Inform patients in advance of the possibility of temporary loss of sensation and muscle function following infiltration and nerve block injections [see ...
-
SPL UNCLASSIFIED SECTIONManufactured by Novocol Pharmaceutical of Canada, Inc. Cambridge, Ontario, Canada N1R 6X3 - Rev. 08/2019 (2306-3) Cook-Waite and Zorcaine are trademarks of Septodont Holdings SAS
-
PRINCIPAL DISPLAY PANEL - 1.7 mL Cartridge Carton50 Single-Dose Cartridges: 1.7 mL each NDC 0362-0830-05 - Cook-Waite® Zorcaine® (articaine HCl and epinephrine) Injection - Articaine hydrochloride 4%(40 mg/mL) and epinephrine ...
-
INGREDIENTS AND APPEARANCEProduct Information