Label: ZONALON- doxepin hydrochloride cream
- NDC Code(s): 0378-8123-30, 0378-8123-45
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 14, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFOR TOPICAL DERMATOLOGIC USE ONLY — NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. Rx Only
-
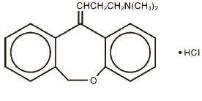
DESCRIPTIONZonalon® (doxepin hydrochloride) Cream, 5% is a topical cream. Each gram contains: 50 mg of doxepin hydrochloride (equivalent to 44.3 mg of doxepin). Doxepin hydrochloride, USP is one of a class ...
-
CLINICAL PHARMACOLOGYAlthough doxepin HCl does have H1 and H2 histamine receptor blocking actions, the exact mechanism by which doxepin exerts its antipruritic effect is unknown. Zonalon® Cream can produce drowsiness ...
-
INDICATIONS AND USAGEZonalon® Cream is indicated for the short-term (up to 8 days) management of moderate pruritus in adult patients with atopic dermatitis or lichen simplex chronicus. (See DOSAGE AND ...
-
CONTRAINDICATIONSBecause doxepin HCl has an anticholinergic effect and because significant plasma levels of doxepin are detectable after topical Zonalon® Cream application, the use of Zonalon® Cream is ...
-
WARNINGSDrowsiness occurs in over 20% of patients treated with Zonalon® Cream, especially in patients receiving treatment to greater than 10% of their body surface area. Patients should be warned about ...
-
PRECAUTIONSGeneral - Drowsiness - Since drowsiness may occur with the use of Zonalon® Cream, patients should be warned of the possibility and cautioned against driving a car or operating dangerous ...
-
ADVERSE REACTIONSControlled Clinical Trials - Systemic Adverse Effects - In controlled clinical trials of patients treated with Zonalon® Cream, the most common systemic adverse event reported was drowsiness ...
-
OVERDOSAGEDeaths may occur from overdosage with this class of drugs. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information ...
-
DOSAGE AND ADMINISTRATIONA thin film of Zonalon® Cream should be applied four times each day with at least a 3 to 4 hour interval between applications. There are no data to establish the safety and effectiveness of ...
-
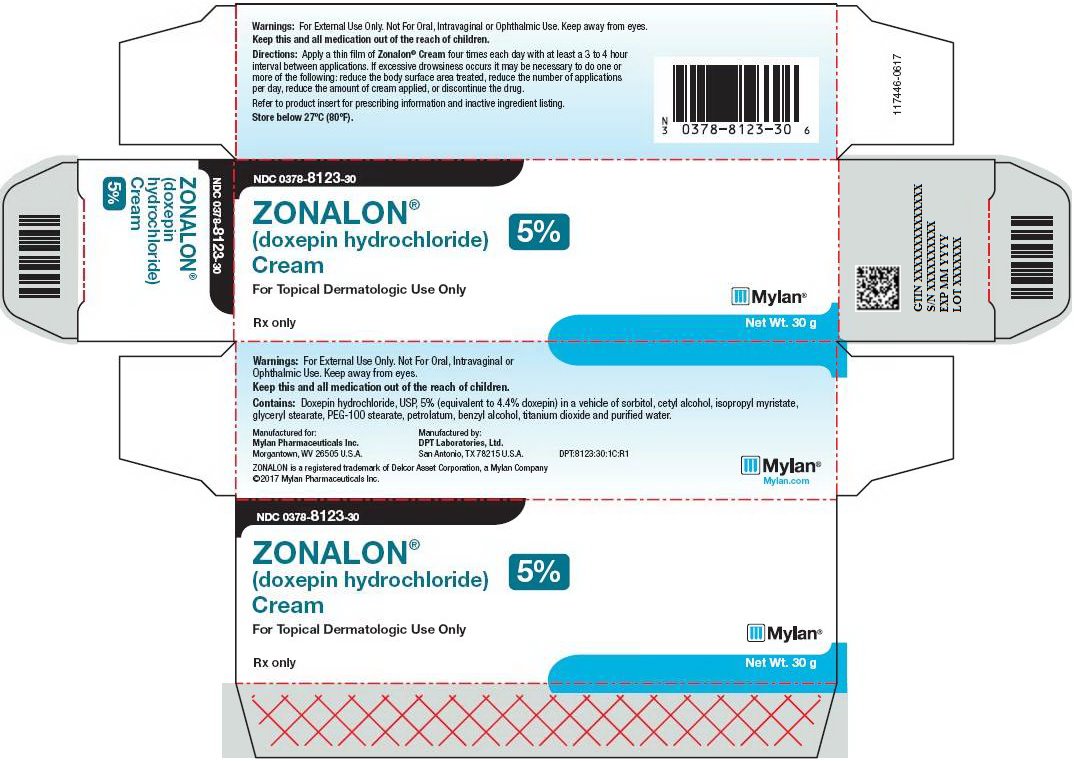
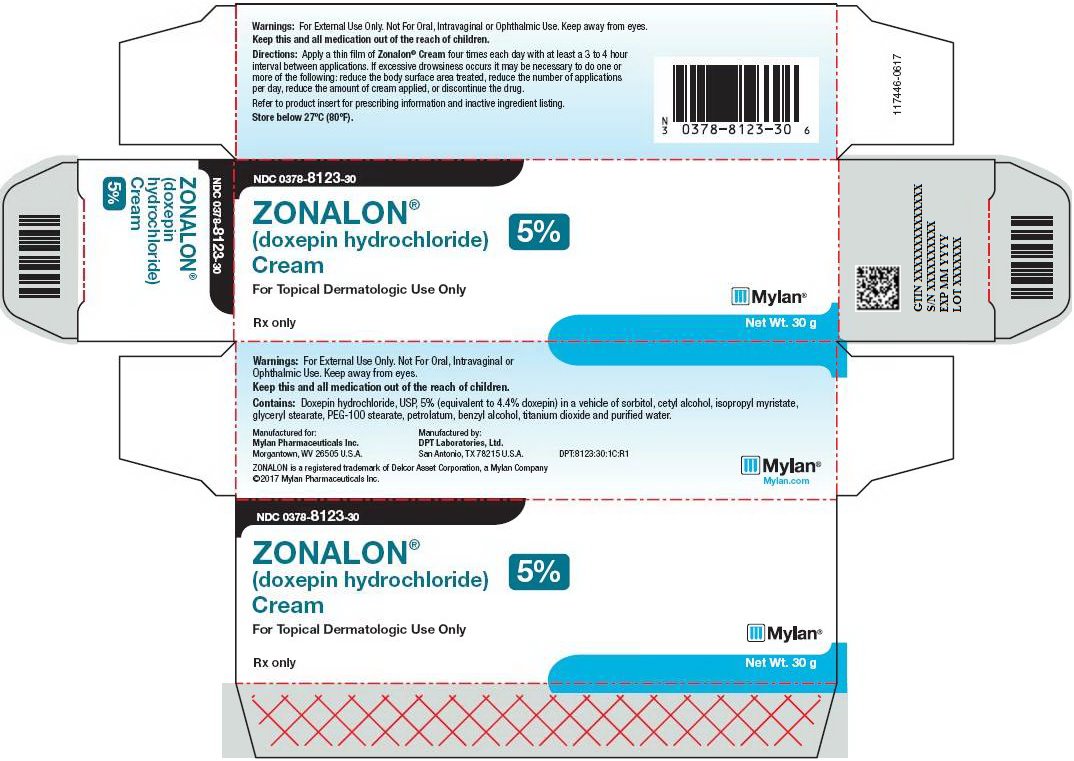
HOW SUPPLIEDZONALON® Cream is available containing 50 mg of doxepin hydrochloride, USP equivalent to 44.3 mg of doxepin. ZONALON® Cream, 5% is a soft white cream available as follows: NDC 0378-8123-30 - carton ...
-
PRINCIPAL DISPLAY PANEL – 5% NDC 0378-8123-30 - ZONALON® (doxepin hydrochloride) Cream 5% For Topical Dermatologic Use Only - Rx only Net Wt. 30 g - Warnings: For External Use Only. Not For Oral, Intravaginal or ...
-
INGREDIENTS AND APPEARANCEProduct Information