Label: ZOLINZA- vorinostat capsule
- NDC Code(s): 0006-0568-40
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZOLINZA safely and effectively. See full prescribing information for ZOLINZA. ZOLINZA® (vorinostat capsules, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZOLINZA® is indicated for the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma who have progressive, persistent or recurrent disease on or following two systemic ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended dose is 400 mg orally once daily with food. Treatment may be continued as long as there is no evidence of progressive disease or unacceptable toxicity ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 100 mg white, opaque, hard gelatin capsules with "568" over "100 mg" printed within radial bar in black ink on the capsule body.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Thromboembolism - Pulmonary embolism occurred in 5% (4/86) of patients receiving ZOLINZA, and deep vein thrombosis has also been reported. Monitor for signs and symptoms of these events ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions have been associated with ZOLINZA in clinical trials and are discussed in greater detail in other sections of the label: Thromboembolism [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Coumarin-Derivative Anticoagulants - Prolongation of prothrombin time (PT) and International Normalized Ratio (INR) were observed in patients receiving ZOLINZA concomitantly with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings from animal studies, ZOLINZA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ...
-
10 OVERDOSAGENo specific information is available on the treatment of overdosage of ZOLINZA. In the event of overdose, it is reasonable to employ the usual supportive measures, e.g., remove unabsorbed ...
-
11 DESCRIPTIONZOLINZA contains vorinostat, which is described chemically as N-hydroxy-N'-phenyloctanediamide. The empirical formula is C14H20N2O3. The molecular weight is 264.32 and the structural formula ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Vorinostat inhibits the enzymatic activity of histone deacetylases HDAC1, HDAC2 and HDAC3 (Class I) and HDAC6 (Class II) at nanomolar concentrations (IC50<86 nM). These ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been performed with vorinostat. Vorinostat was mutagenic in vitro in the bacterial reverse mutation ...
-
14 CLINICAL STUDIESCutaneous T-cell Lymphoma - In two open-label clinical studies, patients with refractory CTCL have been evaluated to determine their response rate to oral ZOLINZA. One study was a single-arm ...
-
15 REFERENCES1. "OSHA Hazardous Drugs." OSHA. [http://www.osha.gov/SLTC/hazardousdrugs/index.html]
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZOLINZA capsules, 100 mg, are white, opaque hard gelatin capsules with "568" over "100 mg" printed within the radial bar in black ink on the capsule body. They are supplied as follows: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Patients should be instructed to drink at least 2 L/day of fluid to prevent dehydration and should promptly ...
-
SPL UNCLASSIFIED SECTIONManuf. for: Merck Sharp & Dohme LLC - Rahway, NJ 07065, USA - Manufactured by: Patheon, Inc. Mississauga, Ontario, Canada L5N 7K9 - For patent information: www.msd.com/research/patent - Copyright ...
-
PATIENT PACKAGE INSERTPatient Information - ZOLINZA® (zo LINZ ah) (vorinostat) capsules - What is ZOLINZA? ZOLINZA is a prescription medicine for a type of cancer called cutaneous T-cell lymphoma (CTCL) ...
-
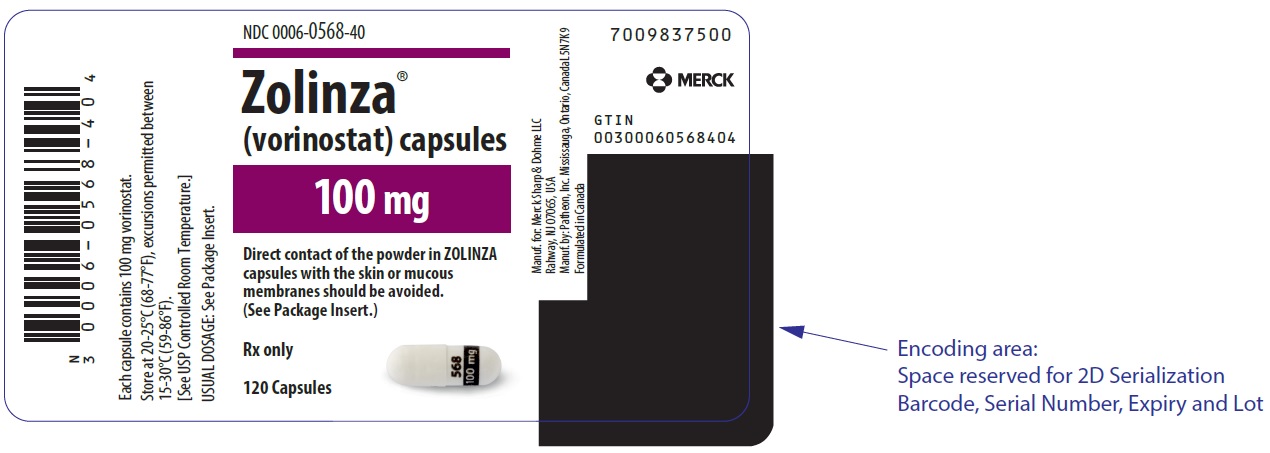
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle LabelNDC 0006-0568-40 - Zolinza® (vorinostat) capsules - 100 mg - Direct contact of the powder in ZOLINZA - capsules with the skin or mucous - membranes should be avoided. (See Package Insert.) Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information