Label: ZIRGAN- ganciclovir gel
- NDC Code(s): 24208-535-15, 24208-535-32, 24208-535-35
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZIRGAN safely and effectively. See full prescribing information for ZIRGAN. ZIRGAN - ®(ganciclovir ophthalmic gel) 0.15 ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZIRGAN - ®(ganciclovir ophthalmic gel) 0.15% is indicated for the treatment of acute herpetic keratitis (dendritic ulcers).
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosing regimen for ZIRGAN is 1 drop in the affected eye 5 times per day (approximately every 3 hours while awake) until the corneal ulcer heals, and then 1 drop 3 times per day for ...
-
3 DOSAGE FORMS AND STRENGTHSZIRGAN contains 0.15% of ganciclovir in a sterile preserved topical ophthalmic gel.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Topical Ophthalmic Use Only - ZIRGAN is indicated for topical ophthalmic use only. 5.2 Avoidance of Contact Lenses - Patients should not wear contact lenses if they have signs or symptoms ...
-
6 ADVERSE REACTIONSMost common adverse reactions reported in patients were blurred vision (60%), eye irritation (20%), punctate keratitis (5%), and conjunctival hyperemia (5%).
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects - Ganciclovir has been shown to be embryotoxic in rabbits and mice following intravenous administration and teratogenic in rabbits. Fetal resorptions were ...
-
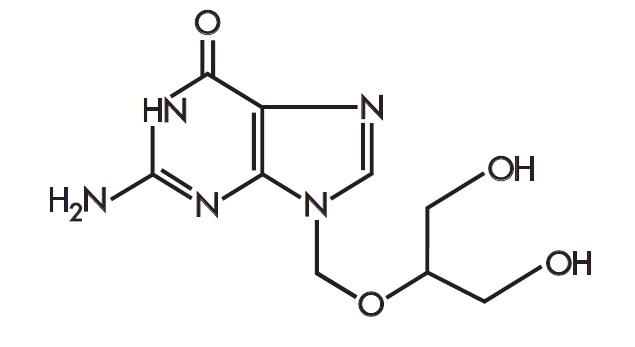
11 DESCRIPTIONZIRGAN - ®(ganciclovir ophthalmic gel) 0.15% contains a sterile, topical antiviral for ophthalmic use. The chemical name is 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine (CAS number ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ZIRGAN contains the active ingredient, ganciclovir, which is a guanosine derivative that, upon phosphorylation, inhibits DNA replication by herpes simplex viruses ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Ganciclovir was carcinogenic in the mouse at oral doses of 20 and 1,000 mg/kg/day (approximately 3,000x and 160,000x the human ocular ...
-
14 CLINICAL STUDIESIn one open-label, randomized, controlled, multicenter clinical trial which enrolled 164 patients with herpetic keratitis, ZIRGAN was non-inferior to acyclovir ophthalmic ointment, 3% in patients ...
-
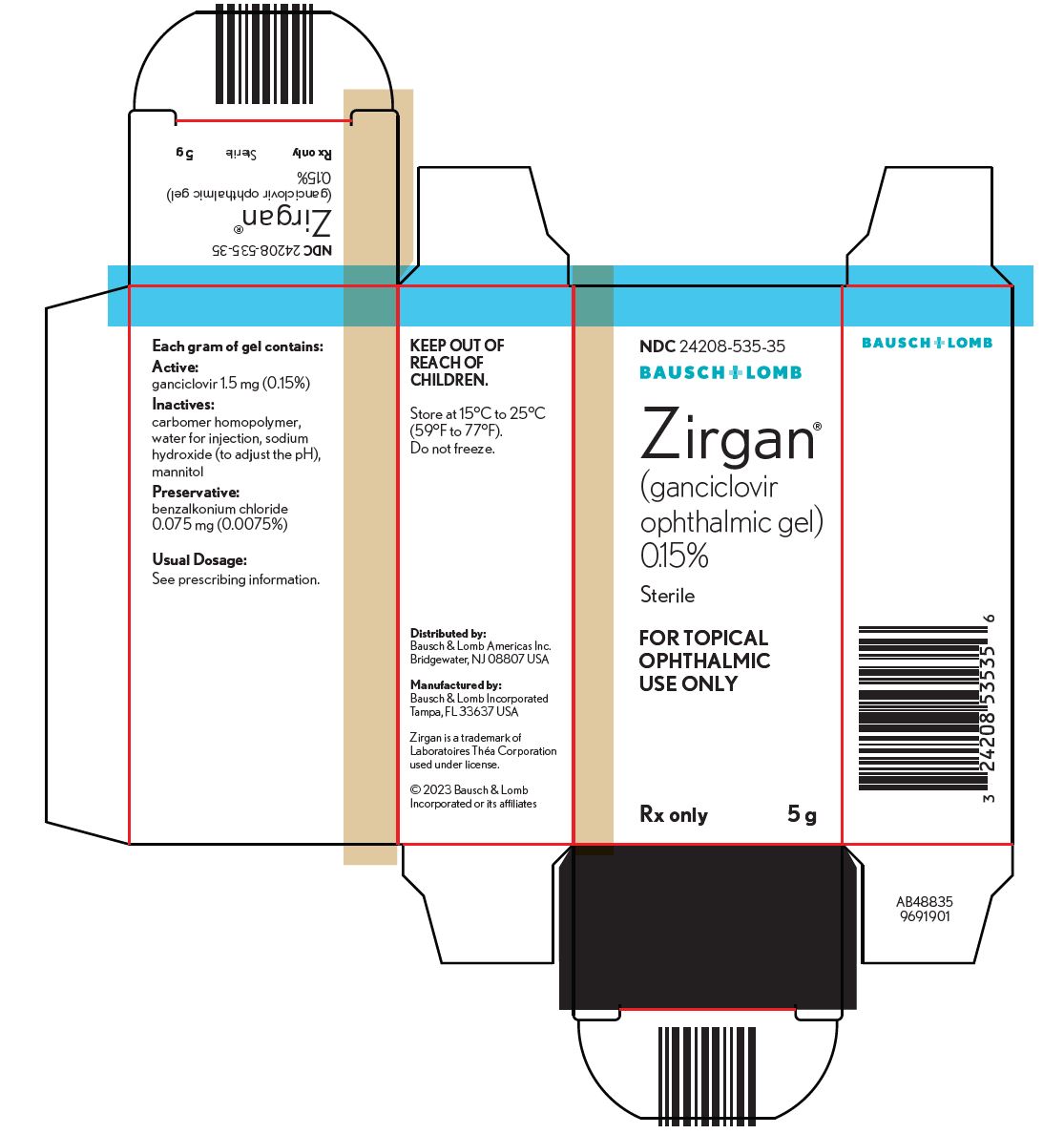
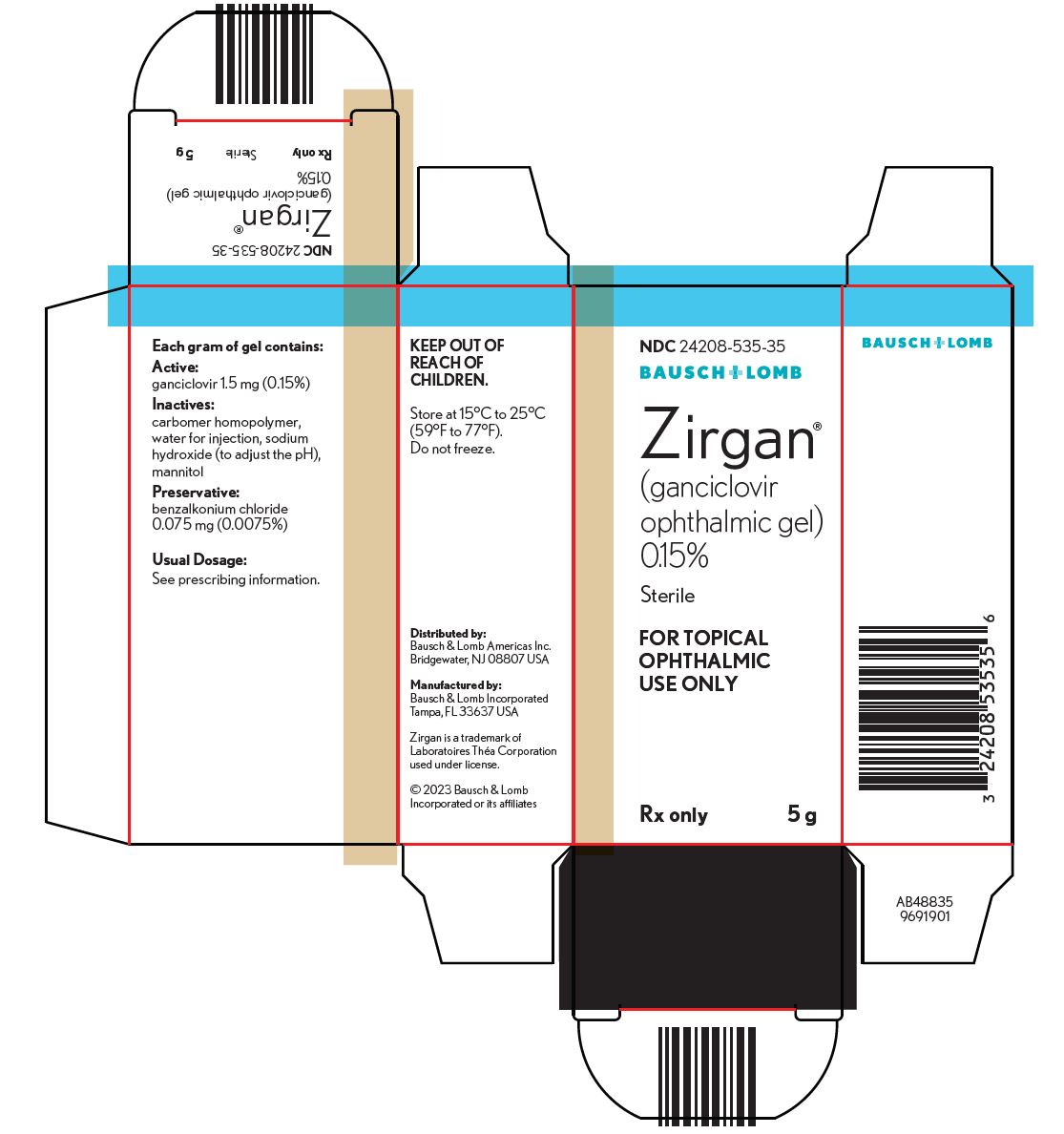
16 HOW SUPPLIED/STORAGE AND HANDLINGZIRGAN - ®is supplied as 5 grams of a sterile, preserved, clear, colorless, topical ophthalmic gel containing 0.15% of ganciclovir in a polycoated aluminum tube with a white polyethylene tip and ...
-
17 PATIENT COUNSELING INFORMATIONThis product is sterile when packaged. Patients should be advised not to allow the dropper tip to touch any surface, as this may contaminate the gel. If pain develops, or if redness, itching, or ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC24208-535-35 - BAUSCH + LOMB - Zirgan - (ganciclovir - ophthalmic gel) 0.15% Sterile - FOR TOPICAL - OPHTHALMIC - USE ONLY - Rx only - 5 g - AB48835 - 9691901
-
INGREDIENTS AND APPEARANCEProduct Information