Label: ZIEXTENZO- pegfilgrastim-bmez injection
- NDC Code(s): 61314-866-01, 61314-866-02
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZIEXTENZO safely and effectively. See full prescribing information for ZIEXTENZO. ZIEXTENZO® (pegfilgrastim-bmez) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Patients with Cancer Receiving Myelosuppressive Chemotherapy - ZIEXTENZO is indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with ...
-
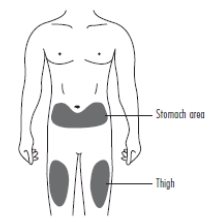
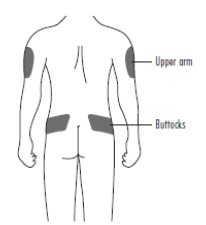
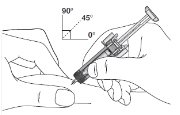
2 DOSAGE AND ADMINISTRATION2.1 Patients with Cancer Receiving Myelosuppressive Chemotherapy - The recommended dosage of ZIEXTENZO is a single subcutaneous injection of 6 mg administered once per chemotherapy cycle. For ...
-
3 DOSAGE FORMS AND STRENGTHSZIEXTENZO is a clear, colorless to slightly yellowish, preservative-free solution available as: • Injection: 6 mg/0.6 mL in a single-dose prefilled syringe for manual use only.
-
4 CONTRAINDICATIONSZIEXTENZO is contraindicated in patients with a history of serious allergic reactions to pegfilgrastim products or filgrastim products. Reactions have included anaphylaxis [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Splenic Rupture - Splenic rupture, including fatal cases, can occur following the administration of pegfilgrastim products. Evaluate for an enlarged spleen or splenic rupture in patients who ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: • Splenic Rupture [see Warnings and Precautions (5.1)] • Acute ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Although available data with ZIEXTENZO or pegfilgrastim product use in pregnant women are insufficient to establish whether there is a drug associated risk of major ...
-
10 OVERDOSAGEOverdosage of pegfilgrastim products may result in leukocytosis and bone pain. Events of edema, dyspnea, and pleural effusion have been reported in a single patient who administered pegfilgrastim ...
-
11 DESCRIPTIONPegfilgrastim-bmez is a covalent conjugate of recombinant methionyl human G-CSF and monomethoxypolyethylene glycol. Recombinant methionyl human G-CSF is a water-soluble 175 amino acid protein with ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pegfilgrastim products are colony-stimulating factors that act on hematopoietic cells by binding to specific cell surface receptors, thereby stimulating proliferation ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or mutagenesis studies have been performed with pegfilgrastim products. Pegfilgrastim did not affect reproductive ...
-
14 CLINICAL STUDIES14.1 Patients with Cancer Receiving Myelosuppressive Chemotherapy - Pegfilgrastim was evaluated in three randomized, double-blind, controlled studies. Studies 1 and 2 were active-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZIEXTENZO single-dose prefilled syringe for manual use - ZIEXTENZO injection is a clear, colorless to slightly yellowish solution supplied in a prefilled single-dose syringe for manual use ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Advise patients of the following risks and potential risks with ZIEXTENZO: • Splenic ...
-
Patient Information
ZIEXTENZO (zee-eks-TEN-zoh) (pegfilgrastim-bmez) injection - Single-dose prefilled syringe - What is ZIEXTENZO? ZIEXTENZO is a man-made form of granulocyte colony-stimulating factor ...
-
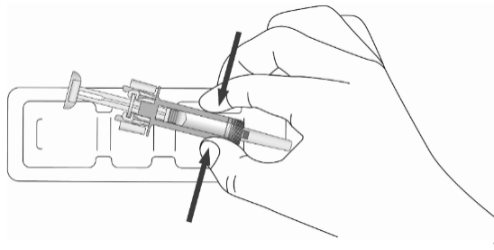
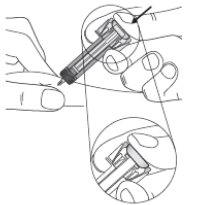
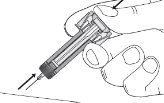
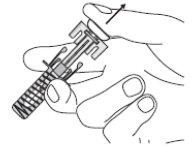
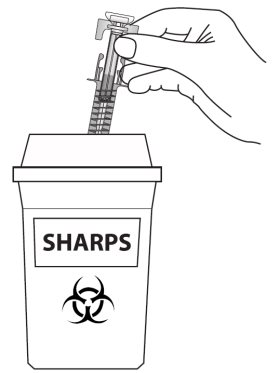
Instructions for Use
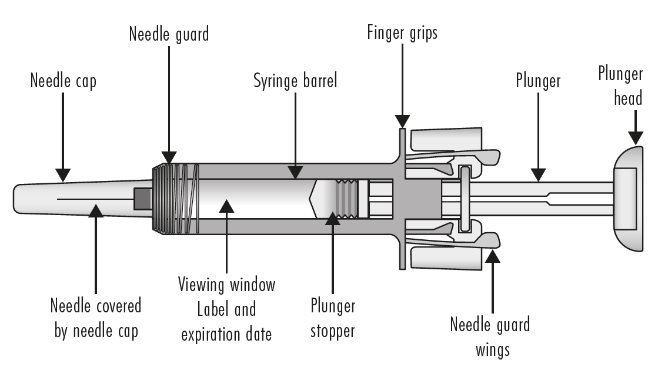
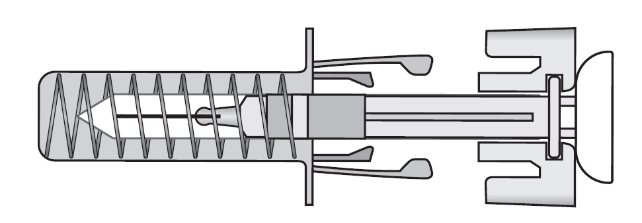
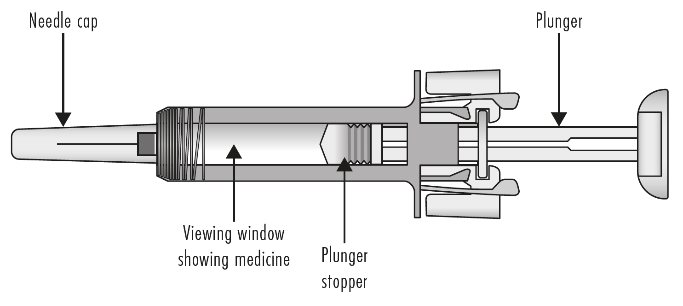
ZIEXTENZO® (pegfilgrastim-bmez) injection - Single-dose prefilled syringe with needle guard - Guide to Parts - Before use - Figure A: Before use. The ZIEXTENZO prefilled syringe with needle guard ...
-
ZIEXTENZO 6 mg/0.6 mL Carton
NDC 61314-866-02 - Ziextenzo®6 mg/0.6 mL - (pegfilgrastim-bmez) Injection - For subcutaneous use only - One Time Use Only - One single-dose prefilled syringe with needle guard - Pegylated Recombinant ...
-
INGREDIENTS AND APPEARANCEProduct Information