Label: PRIALT- ziconotide acetate injection, solution
- NDC Code(s): 70720-720-10, 70720-722-10, 70720-723-10
- Packager: TerSera Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRIALT safely and effectively. See full prescribing information for PRIALT. PRIALT (ziconotide) solution, intrathecal infusion ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: NEUROPSYCHIATRIC ADVERSE REACTIONS
PRIALT is contraindicated in patients with a preexisting history of psychosis. Severe psychiatric symptoms and neurological impairment may occur during treatment with PRIALT. Monitor all patients frequently for evidence of cognitive impairment, hallucinations, or changes in mood or consciousness. Discontinue PRIALT therapy in the event of serious neurological or psychiatric signs or symptoms.
Close -
1 INDICATIONS AND USAGE
PRIALT (ziconotide) solution, intrathecal infusion is indicated for the management of severe chronic pain in adult patients for whom intrathecal therapy is warranted, and who are intolerant of or ...
-
2 DOSAGE AND ADMINISTRATION
2.1 General Information - PRIALT is intended for administration by or under the direction of a physician experienced in the technique of intrathecal administration and who is familiar with the ...
-
3 DOSAGE FORMS AND STRENGTHS
PRIALT (ziconotide) solution, intrathecal infusion is supplied as a 25 mcg/mL concentration in single-use 20 mL glass vials and as a 100 mcg/mL concentration in single-use glass vials containing 1 ...
-
4 CONTRAINDICATIONS
PRIALT is contraindicated in patients with a known hypersensitivity to ziconotide or any of its formulation components. PRIALT is contraindicated in patients with any other concomitant treatment ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Cognitive and Neuropsychiatric Adverse Reactions - Severe psychiatric symptoms and neurological impairment may occur during treatment with PRIALT. PRIALT is contraindicated in patients with ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
Formal PK drug-drug interaction studies have not been performed with PRIALT. As ziconotide is a peptide, it is expected to be completely degraded by endopeptidases and exopeptidases (Phase I ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Available data from postmarketing reports are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal ...
-
10 OVERDOSAGE
The maximum recommended intrathecal PRIALT dose is 19.2 mcg/day. The maximum intrathecal dose of PRIALT in clinical trials was 912 mcg/day. In some patients who received intrathecal doses greater ...
-
11 DESCRIPTION
PRIALT contains ziconotide, a synthetic equivalent of a naturally occurring conopeptide found in the piscivorous marine snail, Conus magus. Ziconotide is a 25 amino acid, polybasic peptide ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Ziconotide binds to N-type calcium channels located on the primary nociceptive (A-δ and C) afferent nerves in the superficial layers (Rexed laminae I and II) of the ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No carcinogenicity studies have been conducted in animals. Mutagenesis - Ziconotide was negative in the in vitro ...
-
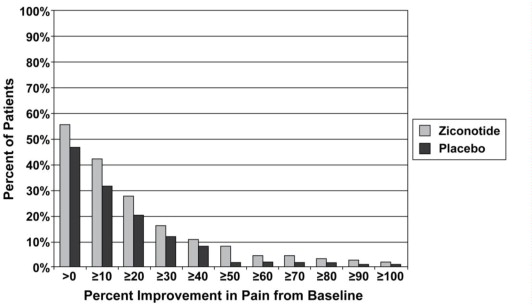
14 CLINICAL STUDIES
The efficacy of intrathecal PRIALT in the management of severe chronic pain was studied in three double-blind, placebo-controlled, multicenter studies in a total of 457 patients (268 PRIALT, 189 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - PRIALT is supplied as a 25 mcg/mL solution in a single-use 20 mL glass vial and as a 100 mcg/mL solution in single-use glass vials containing 1 mL or 5 mL of solution. One ...
-
17 PATIENT COUNSELING INFORMATION
Advise patients that psychiatric symptoms (paranoia, hostility, mania, depressive, suicidal) and cognitive symptoms (confusion, memory problems, speech disorder) may occur during treatment with ...
-
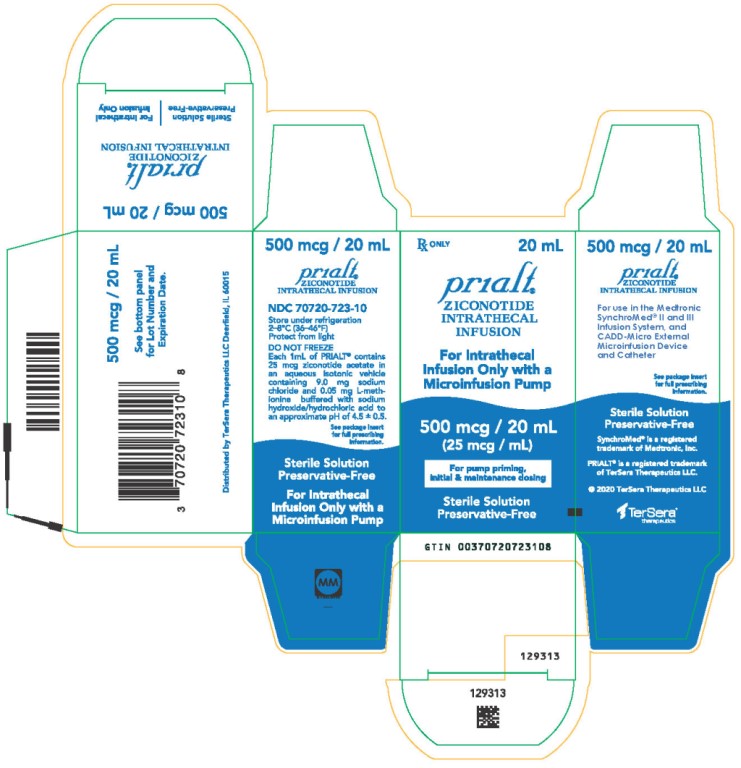
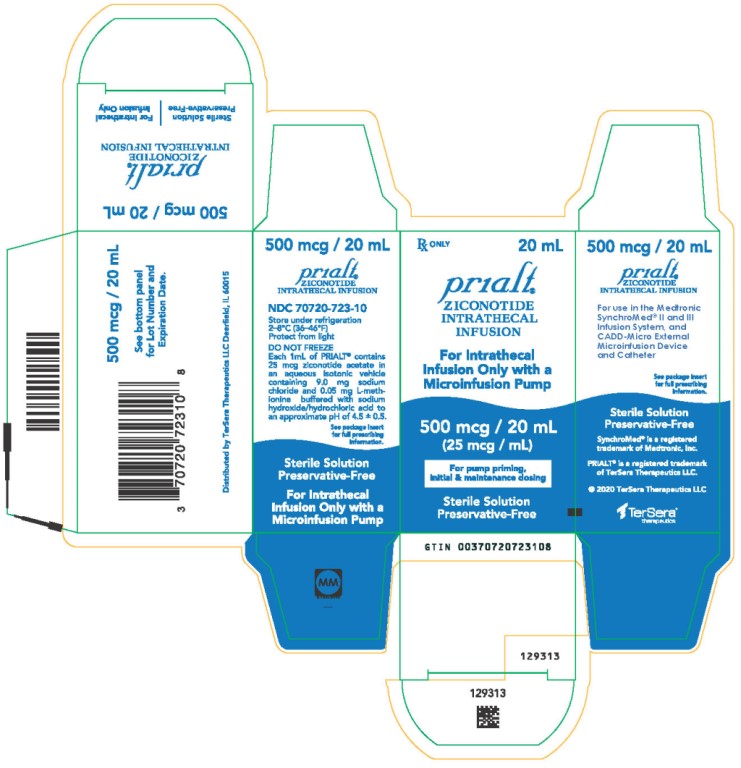
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 20 mL Carton - NDC 70720-723-10 - Principal Display Panel - Rx ONLY 20 mL - Prialt® ZICONOTIDE - INTRATHECAL - INFUSION - For Intrathecal - Infusion Only with a ...
-
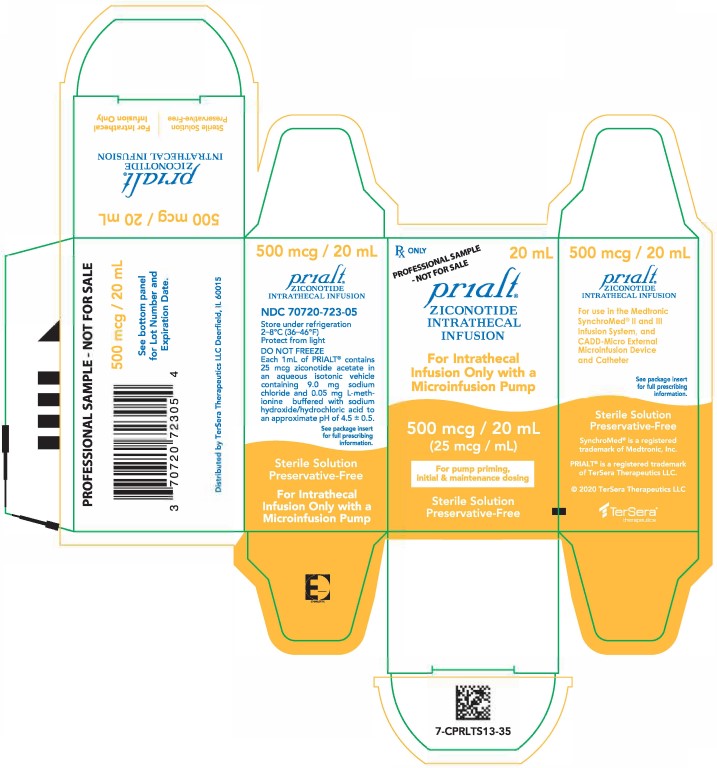
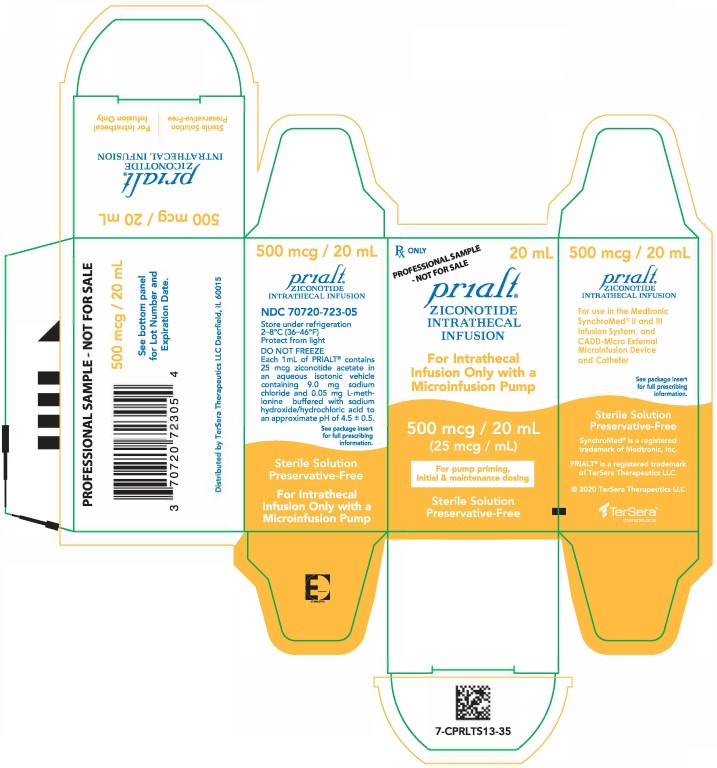
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 20 mL Carton - Sample - NDC 70720-723-05 - Principal Display Panel - Rx ONLY 20 mL - PROFESSIONAL SAMPLE - - NOT FOR SALE - Prialt® ZICONOTIDE - INTRATHECAL ...
-
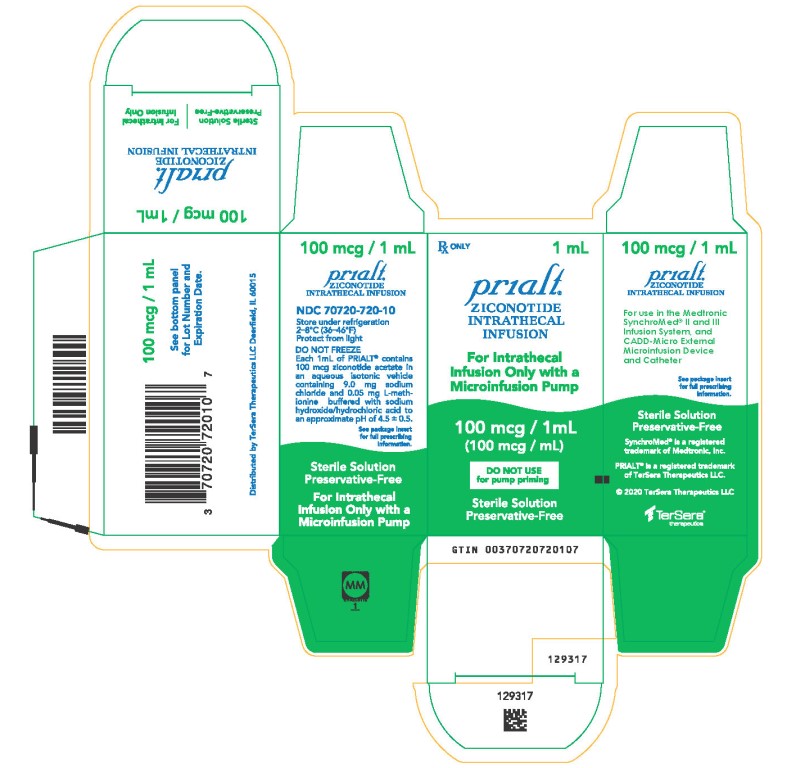
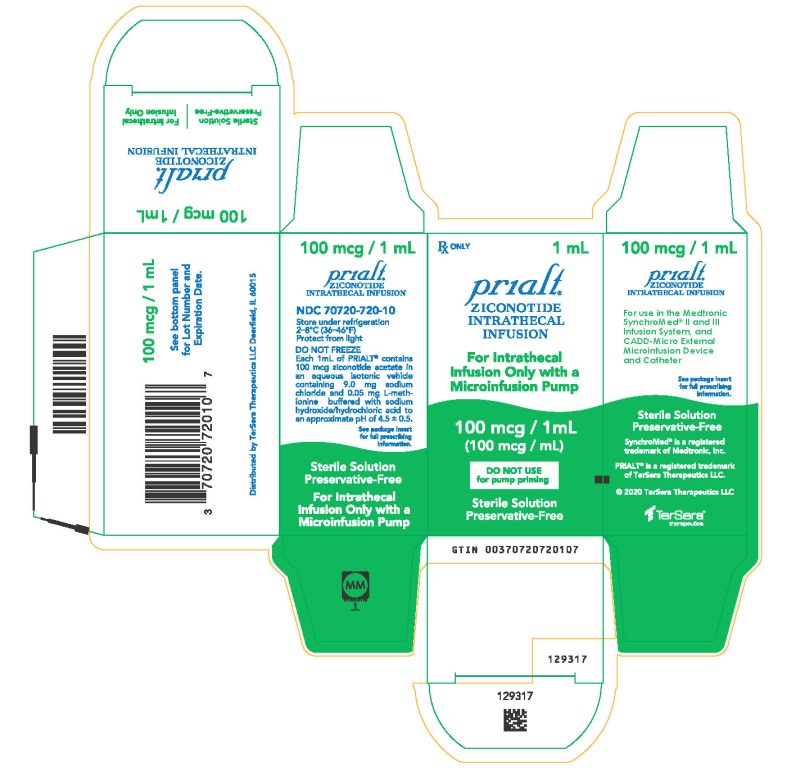
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 1 mL Carton - NDC 70720-720-10 - Principal Display Panel - Rx ONLY 1 mL - Prialt® ZICONOTIDE - INTRATHECAL - INFUSION - For Intrathecal - Infusion Only with a ...
-
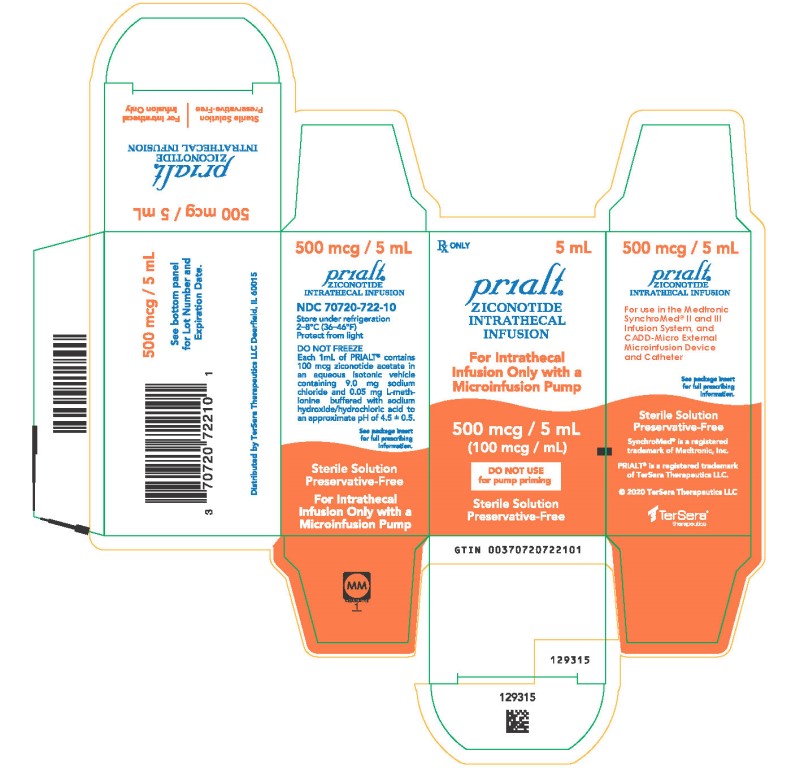
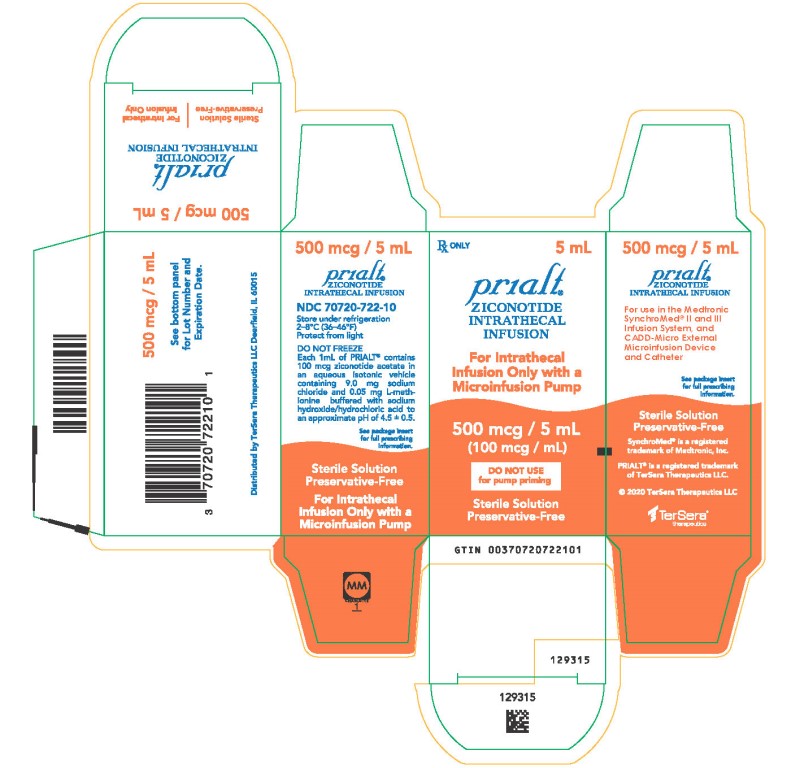
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 5 mL Carton - NDC 70720-722-10 - Principal Display Panel - Rx ONLY 5 mL - Prialt® ZICONOTIDE - INTRATHECAL - INFUSION - For Intrathecal - Infusion Only with a ...
-
INGREDIENTS AND APPEARANCEProduct Information