Label: ZIANA- clindamycin phosphate and tretinoin gel
- NDC Code(s): 99207-300-02, 99207-300-30, 99207-300-60
- Packager: Bausch Health US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 1, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZIANA Gel safely and effectively. See full prescribing information for ZIANA Gel. ZIANA® (clindamycin phosphate 1.2% and tretinoin ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZIANA Gel is indicated for the topical treatment of acne vulgaris in patients 12 years or older.
-
2 DOSAGE AND ADMINISTRATIONAt bedtime, squeeze a pea-sized amount of medication onto one fingertip, dot onto the chin, cheeks, nose, and forehead, then gently rub over the entire face. ZIANA Gel should be kept away from the ...
-
3 DOSAGE FORMS AND STRENGTHSZIANA Gel, a combination of a lincosamide antibiotic and a retinoid, contains clindamycin phosphate 1.2% and tretinoin 0.025%, formulated as a topical gel. Each gram of ZIANA Gel contains, as ...
-
4 CONTRAINDICATIONSZIANA Gel is contraindicated in patients with regional enteritis, ulcerative colitis, or history of antibiotic-associated colitis.
-
5 WARNINGS AND PRECAUTIONS5.1 Colitis - Systemic absorption of clindamycin has been demonstrated following topical use of this product. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under prescribed conditions, adverse reaction rates observed in the clinical trial may not reflect the rates observed in ...
-
7 DRUG INTERACTIONS7.1 Concomitant Topical Medication - Concomitant topical medication, medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect, and products with high ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C. There are no well-controlled trials in pregnant women treated with ZIANA Gel. ZIANA Gel should be used during pregnancy only if the potential benefit ...
-
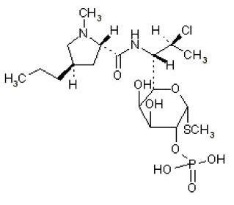
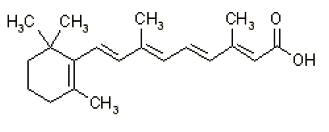
11 DESCRIPTIONZIANA (clindamycin phosphate 1.2% and tretinoin 0.025%) Gel is an antibiotic and retinoid combination gel product with two active ingredients. Clindamycin phosphate is a water-soluble ester of the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanisms of Action - Clindamycin - [See Microbiology (12.4).] Tretinoin - Although the exact mode of action of tretinoin is unknown, current evidence suggests that topical ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity and impairment of fertility testing of ZIANA Gel have not been performed in any ...
-
14 CLINICAL STUDIESThe safety and efficacy of once daily use of ZIANA Gel for treatment of acne vulgaris were assessed in three 12-week prospective, multi-center, randomized, blinded studies in patients 12 years and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZIANA® (clindamycin phosphate 1.2% and tretinoin 0.025%) Gel is supplied as follows: 30 gram tube NDC 99207-300-30 - 60 gram tube NDC 99207-300-60 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Instructions for Use - • At bedtime, the face should be gently washed with a mild soap and warm water. After ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - ZIANA® (ZEE-AH-NA) (clindamycin phosphate 1.2% and tretinoin 0.025%) Gel - IMPORTANT: Not for mouth, eye, or vaginal use. Read the Patient Information that comes with ...
-
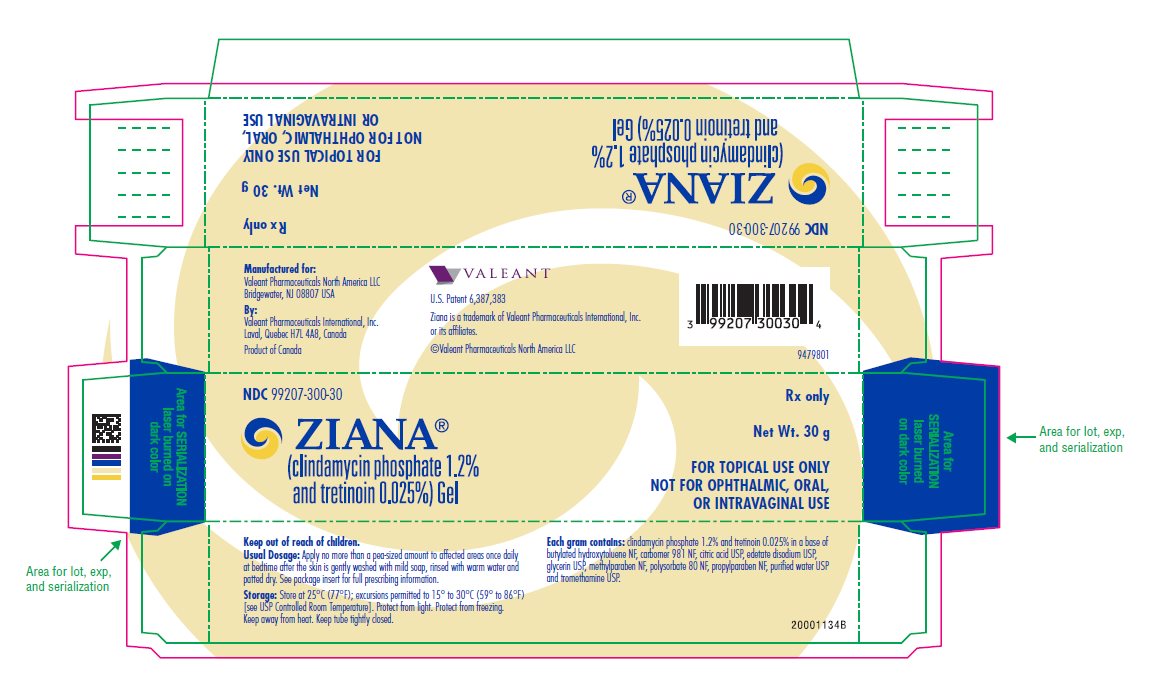
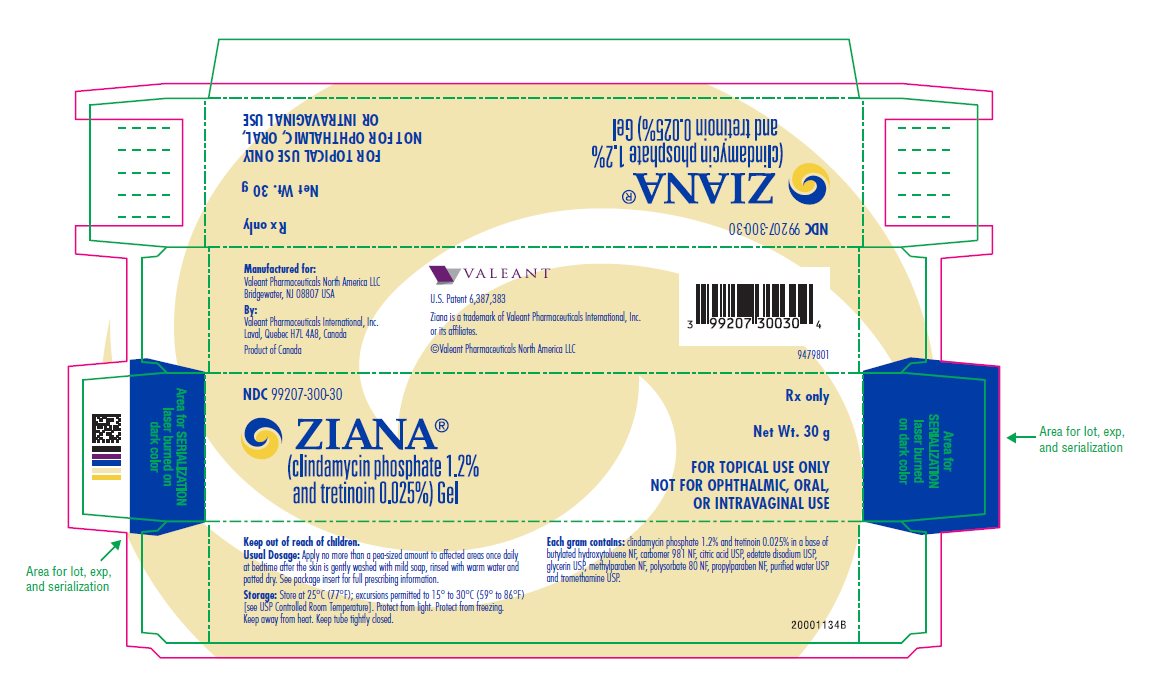
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 30 g Carton - NDC 99207-300-30 - ZIANA® (clindamycin phosphate 1.2% and tretinoin 0.025%) Gel - Rx only - Net Wt. 30 g - FOR TOPICAL USE ONLY - NOT FOR ...
-
INGREDIENTS AND APPEARANCEProduct Information