Label: ZENZEDI- dextroamphetamine sulfate tablet
- NDC Code(s): 24338-850-03, 24338-851-03, 24338-852-03, 24338-853-03, view more
- Packager: AZURITY PHARMACEUTICALS, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
WARNING: ABUSE, MISUSE, AND ADDICTION
Dextroamphetamine sulfate has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including dextroamphetamine sulfate, can result in overdose and death (see OVERDOSAGE), and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing dextroamphetamine sulfate, assess each patient's risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout dextroamphetamine sulfate treatment, reassess each patient's risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction (see WARNINGS and DRUG ABUSE and DEPENDENCE).
Close -
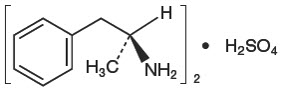
DESCRIPTIONDextroamphetamine sulfate, USP is the dextro isomer of the compound d,l-amphetamine sulfate, a sympathomimetic amine of the amphetamine group. Chemically, dextroamphetamine is ...
-
CLINICAL PHARMACOLOGYAmphetamines are non-catecholamine, sympathomimetic amines with CNS stimulant activity. Peripheral actions include elevations of systolic and diastolic blood pressures and weak bronchodilator and ...
-
INDICATIONS AND USAGEZenzedi® (Dextroamphetamine Sulfate Tablets, USP) is indicated for: 1. Narcolepsy. 2. Attention Deficit Disorder with Hyperactivity, as an integral part of a total treatment program ...
-
CONTRAINDICATIONSKnown hypersensitivity to amphetamine products . During or within 14 days following the administration of monoamine oxidase inhibitors (hypertensive crises may result).
-
WARNINGSAbuse, Misuse, and Addiction - Dextroamphetamine sulfate has a high potential for abuse and misuse. The use of dextroamphetamine sulfate exposes individuals to the risks of abuse and misuse ...
-
PRECAUTIONSInformation for Patients - Advise the patient to read the FDA-approved patient labeling (Medication Guide). Abuse, Misuse, and Addiction - Educate patients and their families about the risks of ...
-
ADVERSE REACTIONSCardiovascular - Palpitations, tachycardia, elevation of blood pressure. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use. Central Nervous ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance - Zenzedi is a Schedule II controlled substance. Abuse - Dextroamphetamine sulfate has a high potential for abuse and misuse which can lead to the development of a ...
-
OVERDOSAGEClinical Effects of Overdose - Overdose of CNS stimulants is characterized by the following sympathomimetic effects: Cardiovascular effects including tachyarrhythmias, and hypertension or ...
-
DOSAGE AND ADMINISTRATIONAmphetamines should be administered at the lowest effective dosage and dosage should be individually adjusted. Late evening doses should be avoided because of the resulting ...
-
HOW SUPPLIEDZenzedi (Dextroamphetamine Sulfate Tablets, USP) are available as: 2.5 mg:White, square tablet, debossed "2.5" on one side and "MIA" on the other side in: Bottles of 30 tablets, NDC ...
-
SPL UNCLASSIFIED SECTIONDEA Order Form Required. [Insert company specific information] Pharmacist: Medication Guide to be dispensed to Patients. Manufactured for: Arbor Pharmaceuticals, LLC - Atlanta, GA 30328 - Rev. ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 10/2023 - MEDICATION GUIDE - Zenzedi® (zen-Zed-ee) (Dextroamphetamine Sulfate Tablets, USP ...
-
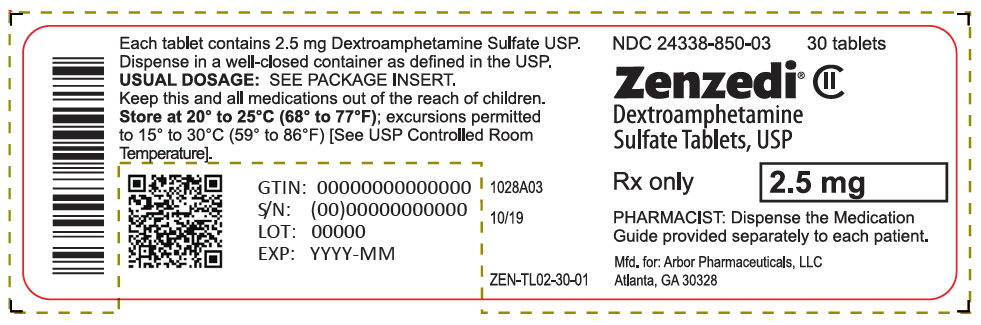
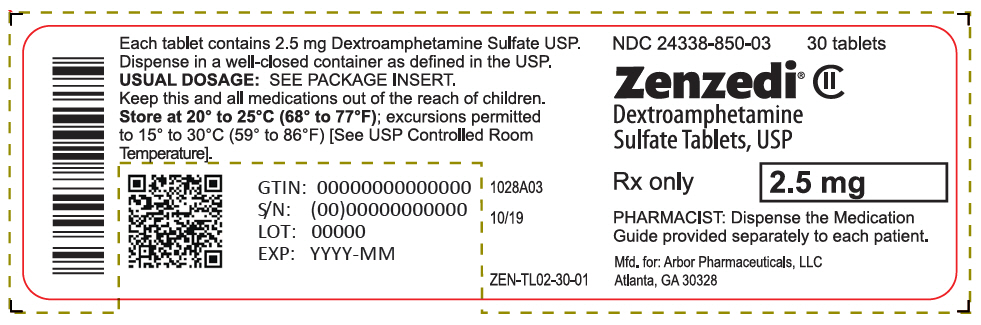
PRINCIPAL DISPLAY PANEL - 2.5 mg Tablet Bottle Label - 24338-850-03NDC 24338-850-03 - 30 tablets - Zenzedi® Dextroamphetamine - Sulfate Tablets, USP - CII - Rx only - 2.5 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient. Mfd. for: Arbor ...
-
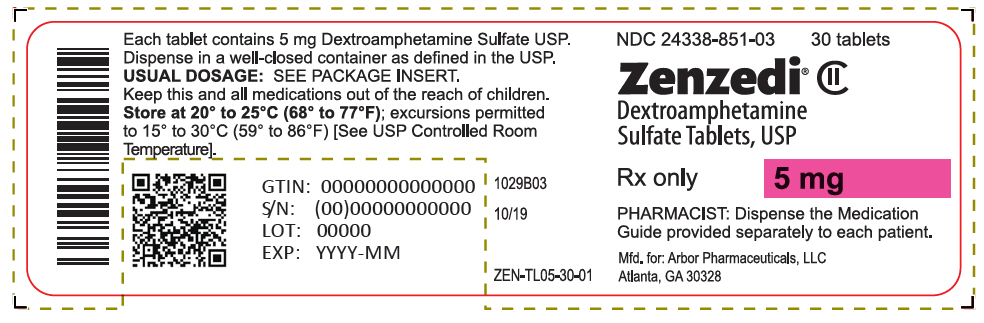
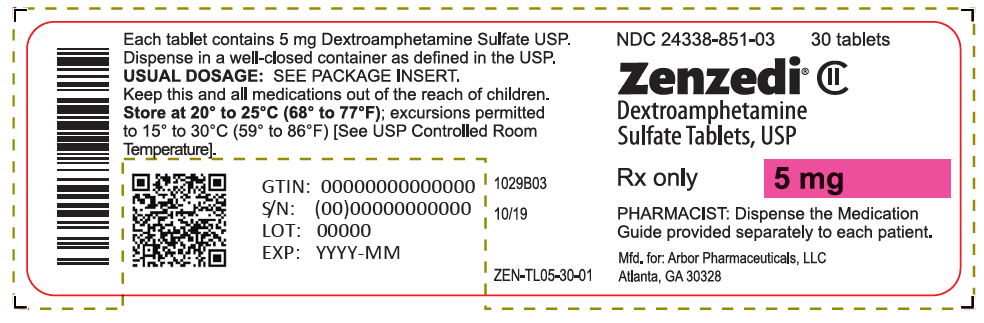
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label - 24338-851-03NDC 24338-851-03 - 30 tablets - Zenzedi® Dextroamphetamine - Sulfate Tablets, USP - CII - Rx only - 5 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient. Mfd. for: Arbor ...
-
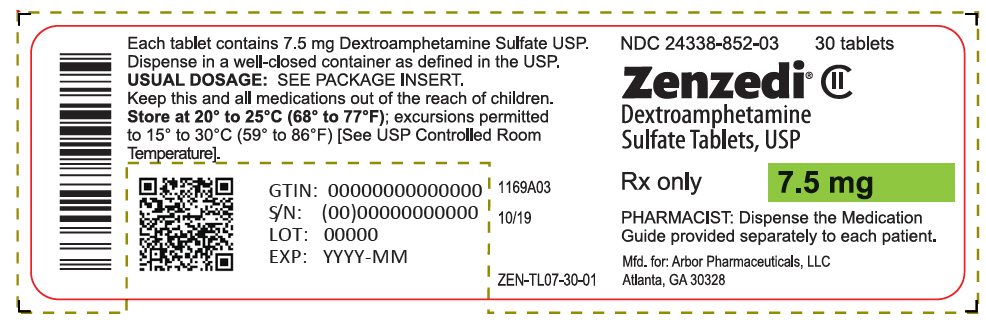
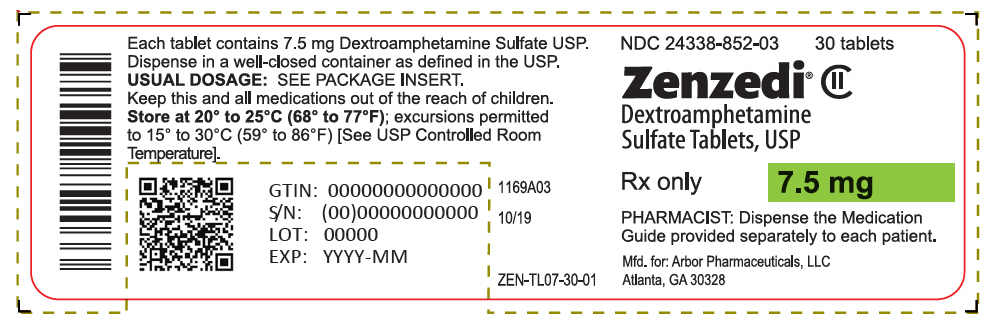
PRINCIPAL DISPLAY PANEL - 7.5 mg Tablet Bottle Label - 24338-852-03NDC 24338-852-03 - 30 tablets - Zenzedi® Dextroamphetamine - Sulfate Tablets, USP - CII - Rx only - 7.5 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient. Mfd. for: Arbor ...
-
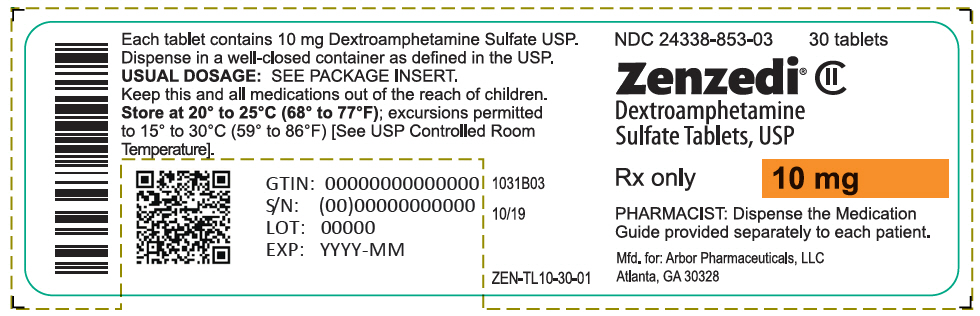
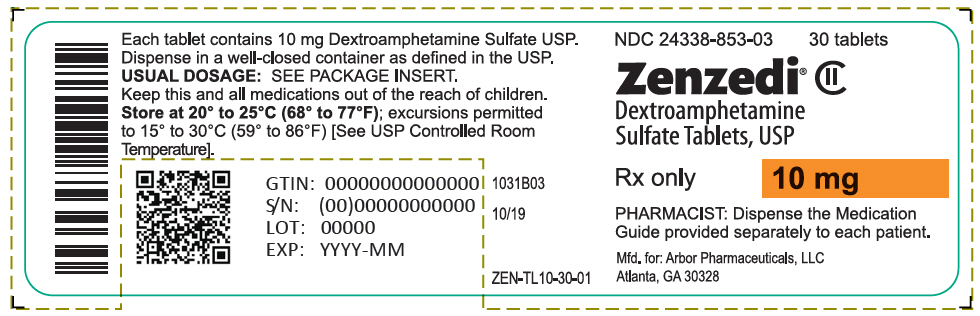
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label - 24338-853-03NDC 24338-853-03 - 30 tablets - Zenzedi® Dextroamphetamine - Sulfate Tablets, USP - CII - Rx only - 10 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient. Mfd. for: Arbor ...
-
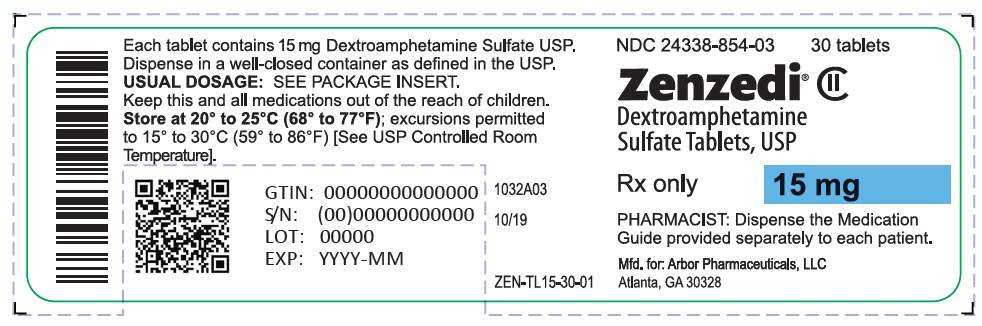
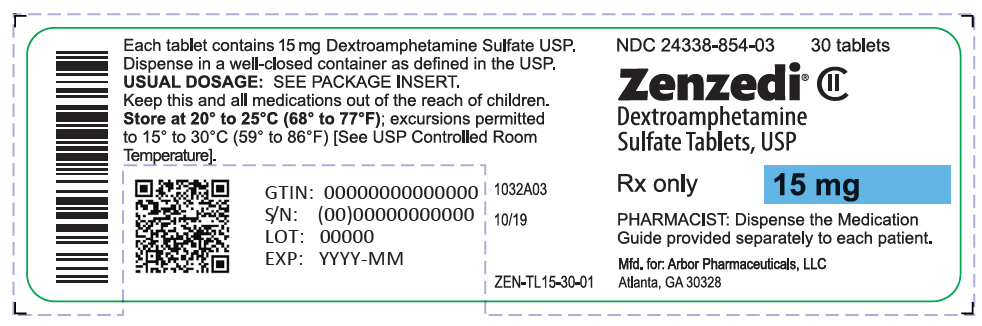
PRINCIPAL DISPLAY PANEL - 15 mg Tablet Bottle Label - 24338-854-03NDC 24338-854-03 - 30 tablets - Zenzedi® Dextroamphetamine - Sulfate Tablets, USP - CII - Rx only - 15 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient. Mfd. for: Arbor ...
-
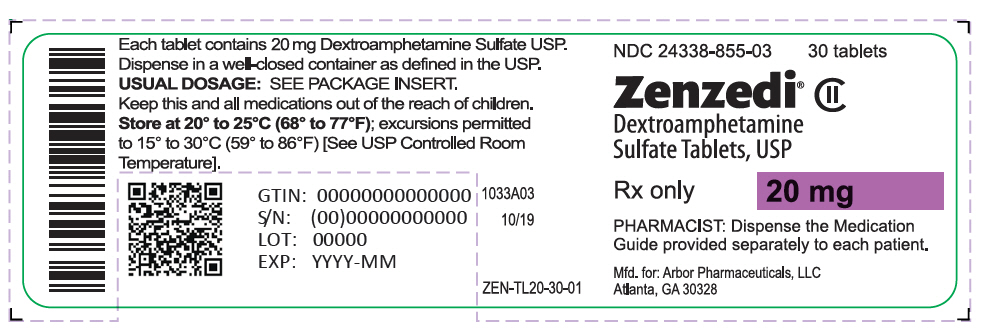
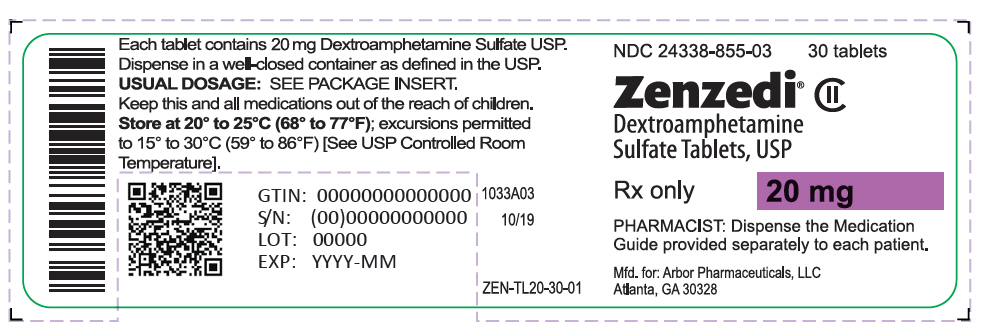
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label - 24338-855-03NDC 24338-855-03 - 30 tablets - Zenzedi® Dextroamphetamine - Sulfate Tablets, USP - CII - Rx only - 20 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient. Mfd. for: Arbor ...
-
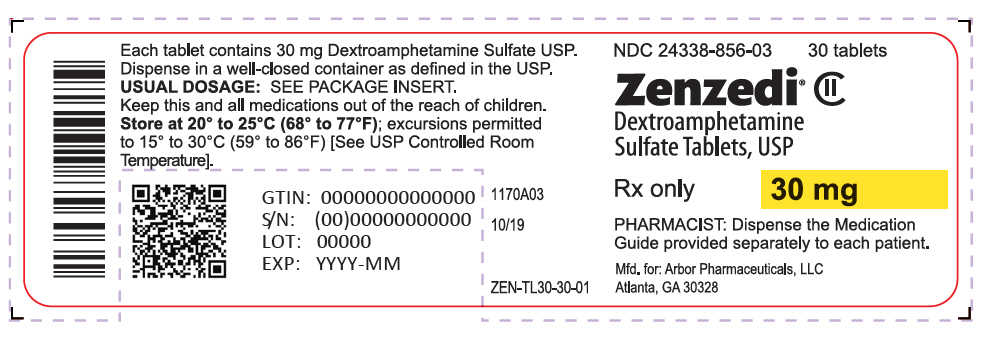
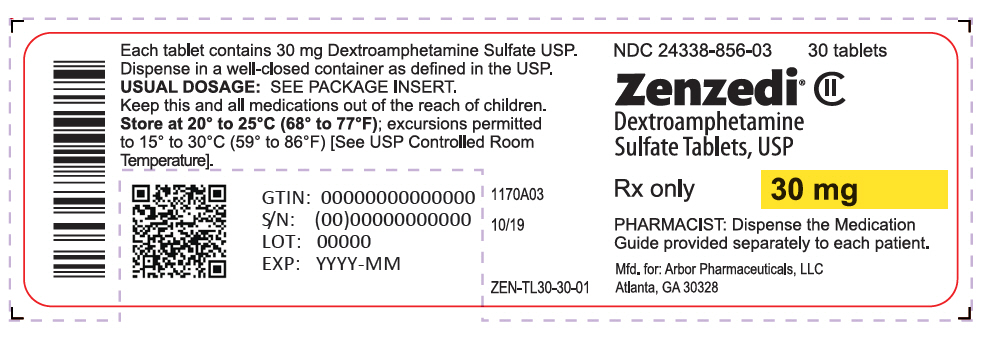
PRINCIPAL DISPLAY PANEL - 30 mg Tablet Bottle Label - 24338-856-03NDC 24338-856-03 - 30 tablets - Zenzedi® Dextroamphetamine - Sulfate Tablets, USP - CII - Rx only - 30 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient. Mfd. for: Arbor ...
-
INGREDIENTS AND APPEARANCEProduct Information