Label: ZELNORM- tegaserod tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 0525-0971-10, 0525-0971-30, 0525-0971-60 - Packager: Alfasigma USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 9, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZELNORM™ safely and effectively. See full prescribing information for ZELNORM™. ZELNORM™ (tegaserod) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
ZELNORM is indicated for the treatment of adult women less than 65 years of age with irritable bowel syndrome with constipation (IBS-C). Limitations of Use - The safety and effectiveness of ...
-
2
DOSAGE AND ADMINISTRATION
The recommended dosage of ZELNORM in adult women less than 65 years of age is 6 mg taken twice daily orally at least 30 minutes before meals [see Clinical Pharmacology (12.3)]. Discontinue ZELNORM ...
-
3
DOSAGE FORMS AND STRENGTHS
ZELNORM Tablets: 6 mg tegaserod; supplied as whitish to slightly yellowish, round flat tablet with a beveled edge engraved with “ZEL” and “6”.
-
4
CONTRAINDICATIONS
ZELNORM is contraindicated in patients with: A history of myocardial infarction (MI), stroke, transient ischemic attack (TIA), or angina [see Warnings and Precautions (5.1)] A history of ...
-
5
WARNINGS AND PRECAUTIONS
5.1 - Cardiovascular Ischemic Events, Including Major Adverse Cardiovascular Events (MACE) Stroke, MI, and cardiovascular death (major adverse cardiovascular events [MACE]) have been ...
-
6
ADVERSE REACTIONS
The following adverse reactions are discussed in more detail elsewhere in the labeling: Cardiovascular Ischemic Events, including MACE [see Warnings and Precautions (5.1)] Ischemic Colitis ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - Available data from case reports with ZELNORM use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or ...
-
10
OVERDOSAGE
Single oral doses of 120 mg (20 times the recommended dose) of ZELNORM were administered to three healthy subjects in one study. All three subjects developed diarrhea and headache. Two of these ...
-
11
DESCRIPTION
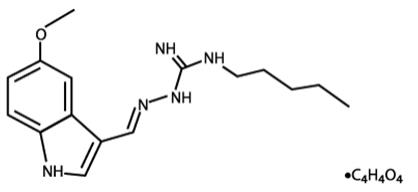
ZELNORM oral tablets contain tegaserod, a serotonin-4 (5-HT4) receptor agonist, as the hydrogen maleate salt. As the maleate salt, tegaserod is chemically designated as ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Tegaserod is an agonist of serotonin type-4 (5-HT4) receptors that stimulates the peristaltic reflex and intestinal secretion, inhibits visceral sensitivity ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Tegaserod was not carcinogenic in rats given oral dietary doses up to 180 mg/kg/day (approximately 93 to 111 times the ...
-
14
CLINICAL STUDIES
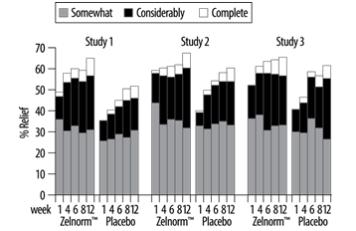
Results in Women - ZELNORM is not recommended in females 65 years of age and older with IBS-C [see Indications and Dosage (1)]. In three multicenter, double-blind, placebo-controlled trials, 2,470 ...
-
16
HOW SUPPLIED / STORAGE AND HANDLING
ZELNORM is supplied as 6 mg tegaserod whitish to slightly yellowish, round flat tablets with a beveled edge engraved with “ZEL” and “6”. Unit Dose (blister pack) Box of 60 (strips of ...
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide). Cardiovascular Ischemic Events, Including MACE - Inform patients that stroke, myocardial infarction, and ...
-
MEDICATION GUIDEMedication Guide - ZELNORMª (ZEL-norm) (tegaserod) tablets, for oral use - What is the most important information I should know about ZELNORM? ZELNORM can cause serious side effects ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 0525-0971-30 - Zelnorm - (tegaserod) tablets - 6 mg per tablet - 30 tablets (3 blister cards of 10 tablets each)
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 0525-0971-60 - Zelnorm - (tegaserod) tablets - 6 mg per tablet - 60 tablets (6 blister cards of 10 tablets each)
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 0525-0971-10 - Zelnorm - (tegaserod) tablets - 6 mg per tablet - 10 tablets (1 blister cards of 10 tablets each)
-
INGREDIENTS AND APPEARANCEProduct Information