Label: ZELBORAF- vemurafenib tablet, film coated
- NDC Code(s): 50242-090-02, 50242-090-86
- Packager: Genentech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZELBORAF safely and effectively. See full prescribing information for ZELBORAF. ZELBORAF® (vemurafenib) tablet for oral use ...These highlights do not include all the information needed to use ZELBORAF safely and effectively. See full prescribing information for ZELBORAF.
ZELBORAF® (vemurafenib) tablet for oral use
Initial U.S. Approval: 2011INDICATIONS AND USAGE
- ZELBORAF® is a kinase inhibitor indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test. (1.1, 2.1)
- ZELBORAF® is indicated for the treatment of patients with Erdheim- Chester Disease with BRAF V600 mutation. (1.2, 2.1)
Limitation of Use: ZELBORAF is not indicated for treatment of patients with wild-type BRAF melanoma (2.1, 5.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablet: 240 mg (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- New Primary Cutaneous Malignancies: Perform dermatologic evaluations prior to initiation of therapy, every 2 months while on therapy, and for up to 6 months following discontinuation of ZELBORAF. Manage with excision and continue treatment without dose adjustment. (5.1)
- New Non-Cutaneous Squamous Cell Carcinoma: Evaluate for symptoms or clinical signs of new non-cutaneous SCC before initiation of treatment and periodically during treatment. (5.1)
- Other Malignancies: Monitor patients receiving ZELBORAF closely for signs or symptoms of other malignancies (5.1).
- Tumor Promotion in BRAF Wild-Type Melanoma: Increased cell proliferation can occur with BRAF inhibitors (5.2).
- Serious Hypersensitivity Reactions including anaphylaxis and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS Syndrome): Discontinue ZELBORAF for severe hypersensitivity reactions. (5.3)
- Severe Dermatologic Reactions, including Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Discontinue ZELBORAF for severe dermatologic reactions. (5.4)
- QT Prolongation: Monitor ECG and electrolytes before and during treatment. Withhold ZELBORAF for QTc of 500 ms or greater. Correct electrolyte abnormalities and control for cardiac risk factors for QT prolongation. (5.5)
- Hepatotoxicity: Measure liver enzymes and bilirubin before initiating ZELBORAF and monitor monthly during treatment. (5.6)
- Photosensitivity: Advise patients to avoid sun exposure. (5.7)
- Serious Ophthalmologic Reactions: Monitor for signs and symptoms of uveitis. (5.8)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of the potential risk to the fetus and to use effective contraception. (5.9, 8.1, 8.3)
- Radiation Sensitization and Radiation Recall: Severe cases have been reported. (5.10).
- Renal Failure: Measure serum creatinine before initiating ZELBORAF and monitor periodically during treatment (5.11).
- Dupuytren's Contracture and plantar fascial fibromatosis: Events should be managed with dose reduction, treatment interruption, or treatment discontinuation. (5.12).
ADVERSE REACTIONS
Melanoma: Most common adverse reactions (≥ 30%) are arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, pruritus, and skin papilloma. (6.1)
Erdheim-Chester Disease: Most common adverse reactions (>50%) are arthralgia, rash maculo-papular, alopecia, fatigue, electrocardiogram QT interval prolonged, and skin papilloma. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Avoid concomitant administration of ZELBORAF with strong CYP3A4 inhibitors or inducers. (7.1)
- CYP1A2 Substrates: ZELBORAF can increase concentrations of CYP1A2 substrates. Avoid concomitant use of ZELBORAF with CYP1A2 substrates with a narrow therapeutic window. If coadministration cannot be avoided, monitor closely for toxicities and consider dose reduction of CYP1A2 substrates. (7.2).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2020
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Unresectable or Metastatic Melanoma
1.2 Erdheim-Chester Disease
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection for Treatment of Melanoma
2.2 Recommended Dose
2.3 Dose Modifications
2.4 Dose Modification for Strong CYP3A4 Inducers
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 New Primary Malignancies
5.2 Tumor Promotion in BRAF Wild-Type Melanoma
5.3 Hypersensitivity Reactions
5.4 Dermatologic Reactions
5.5 QT Prolongation
5.6 Hepatotoxicity
5.7 Photosensitivity
5.8 Ophthalmologic Reactions
5.9 Embryo-Fetal Toxicity
5.10 Radiation Sensitization and Radiation Recall
5.11 Renal Failure
5.12 Dupuytren's Contracture and Plantar Fascial Fibromatosis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Strong CYP3A4 Inhibitors or Inducers on Vemurafenib
7.2 Effect of Vemurafenib on CYP1A2 Substrates
7.3 Concurrent Ipilimumab
7.4 Effect of Vemurafenib on P-gp Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Unresectable or Metastatic Melanoma - ZELBORAF® is indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved ...
1.1 Unresectable or Metastatic Melanoma
ZELBORAF® is indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test.
Limitation of Use: ZELBORAF is not indicated for treatment of patients with wild-type BRAF melanoma [see Warnings and Precautions (5.2)].
Close1.2 Erdheim-Chester Disease
ZELBORAF® is indicated for the treatment of patients with Erdheim-Chester Disease (ECD) with BRAF V600 mutation.
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection for Treatment of Melanoma - Confirm the presence of BRAF V600E mutation in melanoma tumor specimens prior to initiation of treatment with ZELBORAF [see Warnings and ...
2.1 Patient Selection for Treatment of Melanoma
Confirm the presence of BRAF V600E mutation in melanoma tumor specimens prior to initiation of treatment with ZELBORAF [see Warnings and Precautions (5.2)]. Information on FDA-approved tests for the detection of BRAF V600 mutations in melanoma is available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dose
The recommended dose of ZELBORAF is 960 mg (four 240 mg tablets) orally every 12 hours with or without a meal. A missed dose can be taken up to 4 hours prior to the next dose.
Treat patients with ZELBORAF until disease progression or unacceptable toxicity occurs.
Do not take an additional dose if vomiting occurs after ZELBORAF administration, but continue with the next scheduled dose.
Do not crush or chew the tablets.
2.3 Dose Modifications
For Other Adverse Reactions:
Permanently discontinue ZELBORAF for any of the following:
- Grade 4 adverse reaction, first appearance (if clinically appropriate) or second appearance
- QTc prolongation > 500 ms and increased by > 60 ms from pre-treatment values [see Warnings and Precautions (5.5)]
Withhold ZELBORAF for NCI-CTCAE (v4.0) intolerable Grade 2 or greater adverse reactions.
Upon recovery to Grade 0–1, restart ZELBORAF at a reduced dose as follows:
- 720 mg twice daily for first appearance of intolerable Grade 2 or Grade 3 adverse reactions
- 480 mg twice daily for second appearance of Grade 2 (if intolerable) or Grade 3 adverse reactions or for first appearance of Grade 4 adverse reaction (if clinically appropriate)
Do not dose reduce to below 480 mg twice daily.
Close2.4 Dose Modification for Strong CYP3A4 Inducers
Avoid concomitant use of strong CYP3A4 inducers during treatment with ZELBORAF [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. If concomitant use of a strong CYP3A4 inducer is unavoidable, increase the dose of ZELBORAF by 240 mg (one tablet) as tolerated. After discontinuation of a strong CYP3A4 inducer for two weeks, resume the ZELBORAF dose that was taken prior to initiating the strong CYP3A4 inducer.
-
3 DOSAGE FORMS AND STRENGTHSTablet: 240 mg.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 New Primary Malignancies - Cutaneous Malignancies - Cutaneous squamous cell carcinoma, keratoacanthoma, and melanoma occurred at a higher incidence in patients receiving ZELBORAF compared ...
5.1 New Primary Malignancies
Cutaneous Malignancies
Cutaneous squamous cell carcinoma, keratoacanthoma, and melanoma occurred at a higher incidence in patients receiving ZELBORAF compared to those in the control arm in Trial 1. The incidence of cutaneous squamous cell carcinomas (cuSCC) and keratoacanthomas in the ZELBORAF arm was 24% compared to < 1% in the dacarbazine arm [see Adverse Reactions (6.1)]. The median time to the first appearance of cuSCC was 7 to 8 weeks; approximately 33% of patients who developed a cuSCC while receiving ZELBORAF experienced at least one additional occurrence with median time between occurrences of 6 weeks. Potential risk factors associated with cuSCC observed in clinical studies using ZELBORAF included age (≥ 65 years), prior skin cancer, and chronic sun exposure.
In Trial 4, in patients with ECD, the incidence of cuSCC and/or keratoacanthomas was 40.9% (9/22). The median time to first appearance of cuSCC amongst patients with at least one occurrence was 12.1 weeks.
In Trial 1, in patients with unresectable or metastatic melanoma, new primary malignant melanoma occurred in 2.1% (7/336) of patients receiving ZELBORAF compared to none of the patients receiving dacarbazine.
Perform dermatologic evaluations prior to initiation of therapy and every 2 months while on therapy. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Consider dermatologic monitoring for 6 months following discontinuation of ZELBORAF.
Non-Cutaneous Squamous Cell Carcinoma
Non-cutaneous squamous cell carcinomas (non-cuSCC) of the head and neck can occur in patients receiving ZELBORAF [see Adverse Reactions (6.1)]. Monitor patients receiving ZELBORAF closely for signs or symptoms of new non-cuSCC.
Other Malignancies
Based on mechanism of action, ZELBORAF may promote malignancies associated with activation of RAS through mutation or other mechanisms [see Warnings and Precautions (5.2)]. Monitor patients receiving ZELBORAF closely for signs or symptoms of other malignancies.
Cases of myeloid neoplasms amongst patients with ECD have been observed, including in patients who have received ZELBORAF. Monitoring complete blood count in ECD patients with co-existing myeloid malignancies is recommended.
5.2 Tumor Promotion in BRAF Wild-Type Melanoma
In vitro experiments have demonstrated paradoxical activation of MAP-kinase signaling and increased cell proliferation in BRAF wild-type cells that are exposed to BRAF inhibitors. Confirm evidence of BRAF V600E mutation in tumor specimens prior to initiation of ZELBORAF [see Indications and Usage (1) and Dosage and Administration (2.1)].
5.3 Hypersensitivity Reactions
Anaphylaxis and other serious hypersensitivity reactions can occur during treatment and upon re-initiation of treatment with ZELBORAF. Severe hypersensitivity reactions included generalized rash and erythema, hypotension, and drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). Permanently discontinue ZELBORAF in patients who experience a severe hypersensitivity reaction [see Adverse Reactions (6.2)].
5.4 Dermatologic Reactions
Severe dermatologic reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, can occur in patients receiving ZELBORAF. Permanently discontinue ZELBORAF in patients who experience a severe dermatologic reaction [see Adverse Reactions (6.1)].
5.5 QT Prolongation
Concentration-dependent QT prolongation occurred in an uncontrolled, open-label QT sub-study in previously treated patients with BRAF V600E mutation-positive metastatic melanoma [see Clinical Pharmacology (12.2)]. QT prolongation may lead to an increased risk of ventricular arrhythmias, including Torsade de Pointes.
Do not start treatment in patients with uncorrectable electrolyte abnormalities, QTc > 500 ms, or long QT syndrome, or in patients who are taking medicinal products known to prolong the QT interval. Prior to and following treatment initiation or after dose modification of ZELBORAF for QTc prolongation, evaluate ECG and electrolytes (including potassium, magnesium, and calcium) after 15 days, monthly during the first 3 months, and then every 3 months thereafter or more often as clinically indicated.
Withhold ZELBORAF in patients who develop QTc > 500 ms (Grade 3). Upon recovery to QTc ≤ 500 ms (Grade ≤ 2), restart at a reduced dose. Permanently discontinue ZELBORAF treatment if the QTc interval remains > 500 ms and increased > 60 ms from pre-treatment values after controlling cardiac risk factors for QT prolongation (e.g., electrolyte abnormalities, congestive heart failure, and bradyarrhythmias) [see Dosage and Administration (2.3)].
5.6 Hepatotoxicity
Liver injury leading to functional hepatic impairment, including coagulopathy or other organ dysfunction, can occur with ZELBORAF [see Adverse Reactions (6.1)]. Monitor transaminases, alkaline phosphatase, and bilirubin before initiation of treatment and monthly during treatment, or as clinically indicated. Manage laboratory abnormalities with dose reduction, treatment interruption, or treatment discontinuation [see Dosage and Administration (2.3)].
Concurrent Administration with Ipilimumab
The safety and effectiveness of ZELBORAF in combination with ipilimumab have not been established [see Indications and Usage (1)]. In a dose-finding trial, Grade 3 increases in transaminases and bilirubin occurred in a majority of patients who received concurrent ipilimumab (3 mg/kg) and vemurafenib (960 mg BID or 720 mg BID) [see Drug Interactions (7.3)].
5.7 Photosensitivity
Mild to severe photosensitivity can occur in patients treated with ZELBORAF [see Adverse Reactions (6.1)]. Advise patients to avoid sun exposure, wear protective clothing and use a broad spectrum UVA/UVB sunscreen and lip balm (SPF ≥ 30) when outdoors.
Institute dose modifications for intolerable Grade 2 or greater photosensitivity [see Dosage and Administration (2.2)].
5.8 Ophthalmologic Reactions
Uveitis, blurry vision, and photophobia can occur in patients treated with ZELBORAF. In Trial 1, uveitis, including iritis, occurred in 2.1% (7/336) of patients receiving ZELBORAF compared to no patients in the dacarbazine arm. Treatment with steroid and mydriatic ophthalmic drops may be required to manage uveitis. Monitor patients for signs and symptoms of uveitis.
5.9 Embryo-Fetal Toxicity
Based on its mechanism of action, ZELBORAF can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ZELBORAF and for 2 weeks after the final dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
5.10 Radiation Sensitization and Radiation Recall
Radiation sensitization and recall, in some cases severe, involving cutaneous and visceral organs have been reported in patients treated with radiation prior to, during, or subsequent to vemurafenib treatment. Fatal cases have been reported in patients with visceral organ involvement. [see Adverse Reactions (6.2)].
Monitor patients closely when vemurafenib is administered concomitantly or sequentially with radiation treatment.
5.11 Renal Failure
Renal failure, including acute interstitial nephritis and acute tubular necrosis, can occur with ZELBORAF. In Trial 1, in patients with metastatic melanoma, 26% of ZELBORAF-treated patients and 5% of dacarbazine-treated patients experienced Grade 1-2 creatinine elevations [greater than 1 and up to 3 times upper limit of normal (ULN)]; 1.2% of ZELBORAF-treated patients and 1.1% of dacarbazine-treated patients experienced Grade 3-4 creatinine elevations (greater than 3 times ULN).
In Trial 4, in patients with ECD, 86% (19/22) of patients experienced Grade 1/2 creatinine elevations and 9.1% (2/22) of patients experienced Grade 3 creatinine elevations.
Measure serum creatinine before initiation of ZELBORAF and periodically during treatment.
Close5.12 Dupuytren's Contracture and Plantar Fascial Fibromatosis
Dupuytren's contracture and plantar fascial fibromatosis have been reported with ZELBORAF. The majority of cases were mild to moderate, but severe, disabling cases of Dupuytren's contracture have also been reported [see Dosage and Administration (2.3), Adverse Reactions (6.1, 6.2)].
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the label: New Primary Malignancies [see Warnings and Precautions (5.1)] Hypersensitivity Reactions [see ...
The following adverse reactions are discussed in greater detail in other sections of the label:
- New Primary Malignancies [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Dermatologic Reactions [see Warnings and Precautions (5.4)]
- QT Prolongation [see Warnings and Precautions (5.5)]
- Hepatotoxicity [see Warnings and Precautions (5.6)]
- Photosensitivity [see Warnings and Precautions (5.7)]
- Ophthalmologic Reactions [see Warnings and Precautions (5.8)]
- Radiation Sensitization and Radiation Recall [see Warnings and Precautions (5.10)]
- Renal Failure [see Warnings and Precautions (5.11)]
- Dupuytren's Contracture and Plantar Fascial Fibromatosis [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not predict the rates observed in a broader patient population in clinical practice.
Unresectable or Metastatic Melanoma with BRAF V600E Mutation This section describes adverse drug reactions (ADRs) identified from analyses of Trial 1 and Trial 2 [see Clinical Studies (14)]. Trial 1 randomized (1:1) 675 treatment-naive patients with unresectable or metastatic melanoma to receive ZELBORAF 960 mg orally twice daily or dacarbazine 1000 mg/m2 intravenously every 3 weeks. In Trial 2, 132 patients with metastatic melanoma and failure of at least one prior systemic therapy received treatment with ZELBORAF 960 mg orally twice daily.
Table 1 presents adverse reactions reported in at least 10% of unresectable or metastatic melanoma patients treated with ZELBORAF. The most common adverse reactions of any grade (≥ 30% in either study) in ZELBORAF-treated patients were arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, pruritus, and skin papilloma. The most common (≥ 5%) Grade 3 adverse reactions were cuSCC and rash. The incidence of Grade 4 adverse reactions was ≤ 4% in both studies.
The incidence of adverse events resulting in permanent discontinuation of study medication in Trial 1 was 7% for the ZELBORAF arm and 4% for the dacarbazine arm. In Trial 2, the incidence of adverse events resulting in permanent discontinuation of study medication was 3% in ZELBORAF-treated patients. The median duration of study treatment was 4.2 months for ZELBORAF and 0.8 months for dacarbazine in Trial 1, and 5.7 months for ZELBORAF in Trial 2.
Table 1 Adverse Reactions Reported in ≥ 10% of Unresectable or Metastatic Melanoma Patients Treated with ZELBORAF* ADRs Trial 1: Treatment-Naïve Patients Trial 2: Patients with Failure of at Least One Prior Systemic Therapy ZELBORAF
n=336Dacarbazine
n=287ZELBORAF
n=132All Grades
(%)Grade 3†
(%)All Grades
(%)Grade 3
(%)All Grades (%) Grade 3† (%) - *
- Adverse drug reactions, reported using MedDRA and graded using NCI-CTC-AE v 4.0 (NCI common toxicity criteria) for assessment of toxicity.
- †
- Grade 4 adverse reactions limited to gamma-glutamyltransferase increased (< 1% in Trial 1 and 4% in Trial 2).
- ‡
- Includes both squamous cell carcinoma of the skin and keratoacanthoma.
- §
- Cases of cutaneous squamous cell carcinoma were required to be reported as Grade 3 per protocol.
Skin and subcutaneous tissue disorders Rash 37 8 2 0 52 7 Photosensitivity reaction 33 3 4 0 49 3 Alopecia 45 < 1 2 0 36 0 Pruritus 23 1 1 0 30 2 Hyperkeratosis 24 1 < 1 0 28 0 Rash maculo-papular 9 2 < 1 0 21 6 Actinic keratosis 8 0 3 0 17 0 Dry skin 19 0 1 0 16 0 Rash papular 5 < 1 0 0 13 0 Erythema 14 0 2 0 8 0 Musculoskeletal and connective tissue disorders Arthralgia 53 4 3 < 1 67 8 Myalgia 13 < 1 1 0 24 < 1 Pain in extremity 18 < 1 6 2 9 0 Musculoskeletal pain 8 0 4 < 1 11 0 Back pain 8 < 1 5 < 1 11 < 1 General disorders and administration site conditions Fatigue 38 2 33 2 54 4 Edema peripheral 17 < 1 5 0 23 0 Pyrexia 19 < 1 9 < 1 17 2 Asthenia 11 < 1 9 < 1 2 0 Gastrointestinal disorders Nausea 35 2 43 2 37 2 Diarrhea 28 < 1 13 < 1 29 < 1 Vomiting 18 1 26 1 26 2 Constipation 12 < 1 24 0 16 0 Nervous system disorders Headache 23 < 1 10 0 27 0 Dysgeusia 14 0 3 0 11 0 Neoplasms benign, malignant and unspecified (includes cysts and polyps) Skin papilloma 21 < 1 0 0 30 0 Cutaneous SCCठ24 22 < 1 < 1 24 24 Seborrheic keratosis 10 < 1 1 0 14 0 Investigations Gamma-glutamyltransferase increased 5 3 1 0 15 6 Metabolism and nutrition disorders Decreased appetite 18 0 8 < 1 21 0 Respiratory, thoracic and mediastinal disorders Cough 8 0 7 0 12 0 Injury, poisoning and procedural complications Sunburn 10 0 0 0 14 0 Clinically relevant adverse reactions reported in < 10% of unresectable or metastatic melanoma patients treated with ZELBORAF in the Phase 2 and Phase 3 studies include:
Skin and subcutaneous tissue disorders: palmar-plantar erythrodysesthesia syndrome, keratosis pilaris, panniculitis, erythema nodosum, Stevens-Johnson syndrome, toxic epidermal necrolysis
Musculoskeletal and connective tissue disorders: arthritis, Dupuytren's contracture
Nervous system disorders: neuropathy peripheral, VIIth nerve paralysis
Neoplasms benign, malignant and unspecified (includes cysts and polyps): basal cell carcinoma, oropharyngeal squamous cell carcinoma
Infections and infestations: folliculitis
Eye disorders: retinal vein occlusion
Vascular disorders: vasculitis
Cardiac disorders: atrial fibrillation
Table 2 shows the incidence of worsening liver laboratory abnormalities in Trial 1 summarized as the proportion of patients who experienced a shift from baseline to Grade 3 or 4.
Table 2 Change from Baseline to Grade 3/4 Liver Laboratory Abnormalities in Trial 1* Parameter Change From Baseline to Grade 3/4 ZELBORAF (%) Dacarbazine (%) - *
- For ALT, alkaline phosphatase, and bilirubin, there were no patients with a change to Grade 4 in either treatment arm.
GGT 11.5 8.6 AST 0.9 0.4 ALT 2.8 1.9 Alkaline phosphatase 2.9 0.4 Bilirubin 1.9 0 Erdheim-Chester Disease (ECD)
This section describes adverse reactions identified from analyses of Trial 4 [see Clinical Studies (14)]. In Trial 4, 22 patients with BRAF V600 mutation-positive ECD received ZELBORAF 960 mg twice daily.
The median treatment duration for ECD patients in this study was 14.2 months. Table 3 presents adverse reactions reported in at least 20% of BRAF V600 mutation-positive ECD patients treated with ZELBORAF.
In Trial 4, the most commonly reported adverse reactions (> 50%) in patients with BRAF V600 mutation- positive ECD treated with ZELBORAF were arthralgia, rash maculo-papular, alopecia, fatigue, electrocardiogram QT interval prolonged, and skin papilloma. The most common (≥ 10%) Grade ▯ 3 adverse reactions were squamous cell carcinoma of the skin, hypertension, rash maculo-papular, and arthralgia.
The incidence of adverse reactions resulting in permanent discontinuation of study medication was 32%.
Table 3 Adverse Reactions Reported in ≥ 20% of ECD Patients Treated with ZELBORAF* Trial 4: Patients with ECD n=22 Body System
Adverse ReactionsAll Grades (%) Grade 3-4 (%) Skin and subcutaneous tissue disorders Rash maculo-papular 59 18 Alopecia 55 - Hyperkeratosis 50 5 Dry skin 45 - Photosensitivity reaction 41 - Palmar-plantar erythrodysaesthesia syndrome 41 - Pruritus 36 - Actinic keratosis 32 5 Keratosis pilaris 32 - Rash papular 23 - Musculoskeletal and connective tissue disorders Arthralgia 82 14 General disorders and administration site conditions Fatigue 55 5 Gastrointestinal disorders Diarrhea 50 - Nausea 32 - Vomiting 23 - Nervous system disorders Peripheral sensory neuropathy 36 - Neoplasms benign, malignant and unspecified (incl. cysts and polyps) Skin papilloma 55 - Seborrhoeic keratosis 41 - SCC of skin† 36 36 Melanocytic nevus 23 _ Cardiac disorders Electrocardiogram QT interval prolonged 55 5 Respiratory, thoracic and mediastinal disorders Cough 36 - Vascular disorders Hypertension 36 23 Injury, poisoning and procedural complications Sunburn 23 - Clinically relevant adverse reactions reported in < 20% of ECD patients treated with ZELBORAF in Trial 4 include:
Neoplasms benign, malignant and unspecified (includes cysts and polyps): keratoacanthoma
Musculoskeletal and connective tissue disorders: Dupuytren's contracture
Table 4 shows the incidence of worsening liver laboratory abnormalities in Trial 4 summarized as the proportion of ECD patients who experienced a shift from baseline to Grade 3 or 4.
Table 4 Change from Baseline to Grade 3 Liver Laboratory Abnormalities in Trial 4 Change From Baseline to Grade 3 Parameter Vemurafenib (%) AST 0 ALT 9.1 Alkaline phosphatase 4.5 Bilirubin 0 Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ZELBORAF. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Neoplasms benign, malignant and unspecified (incl. cysts and polyps): Progression of pre-existing chronic myelomonocytic leukemia with NRAS mutation [see Warnings and Precautions (5.1)].
Skin and subcutaneous tissue disorders: Drug reaction with eosinophilia and systemic symptoms (DRESS syndrome) [see Warnings and Precautions (5.3)].
Blood and lymphatic systems disorder: Neutropenia
Injury, poisoning and procedural complications: Radiation sensitization and recall [see Warnings and Precautions (5.10)].
Gastrointestinal disorders: Pancreatitis
Renal and urinary disorders: Acute interstitial nephritis, acute tubular necrosis [see Warnings and Precautions (5.11)].
Musculoskeletal and connective tissue disorders: Dupuytren's contracture and plantar fascial fibromatosis [see Warnings and Precautions (5.12)].
-
7 DRUG INTERACTIONS7.1 Effect of Strong CYP3A4 Inhibitors or Inducers on Vemurafenib - Strong CYP3A4 Inhibitors - Coadministration of a strong CYP3A4 inhibitor increased vemurafenib plasma concentrations and may ...
7.1 Effect of Strong CYP3A4 Inhibitors or Inducers on Vemurafenib
Strong CYP3A4 Inhibitors
Coadministration of a strong CYP3A4 inhibitor increased vemurafenib plasma concentrations and may lead to increased toxicity. Avoid coadministration of ZELBORAF with strong CYP3A4 inhibitors. If coadministration of a strong CYP3A4 inhibitor is unavoidable, consider dose reduction of ZELBORAF, if clinically indicated. [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
Strong CYP3A4 Inducers
Coadministration of ZELBORAF with rifampin, a strong CYP3A4 inducer, decreased vemurafenib plasma concentrations and may result in decreased efficacy. Avoid coadministration of ZELBORAF with strong CYP3A4 inducers (e.g., phenytoin, carbamazepine, rifampin), and replace these drugs with alternative drugs when possible. If coadministration of a strong CYP3A4 inducer is unavoidable, increase the dose of ZELBORAF by 240 mg (one tablet) as tolerated [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
7.2 Effect of Vemurafenib on CYP1A2 Substrates
Coadministration of ZELBORAF with tizanidine, a sensitive CYP1A2 substrate, increased tizanidine systemic exposure by 4.7-fold. Avoid concomitant use of ZELBORAF with drugs having a narrow therapeutic window that are predominantly metabolized by CYP1A2 [see Clinical Pharmacology (12.3)]. If coadministration cannot be avoided, monitor closely for toxicities and consider a dose reduction of concomitant CYP1A2 substrates.
7.3 Concurrent Ipilimumab
Increases in transaminases and bilirubin occurred in a majority of patients who received concurrent ipilimumab and ZELBORAF [see Warnings and Precautions Section 5.6].
Close7.4 Effect of Vemurafenib on P-gp Substrates
Coadministration of ZELBORAF with digoxin, a sensitive P-glycoprotein (P-gp) substrate, increased digoxin systemic exposure by 1.8-fold. Avoid concurrent use of P-gp substrates known to have narrow therapeutic indices. If use of these medications is unavoidable, consider dose reduction of P-gp substrates with narrow therapeutic indices.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, ZELBORAF can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available ...
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, ZELBORAF can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of ZELBORAF in pregnant women to determine the drug-associated risk; however, placental transfer of vemurafenib to a fetus has been reported. Exposure to vemurafenib could not be achieved in animals at levels sufficient to fully address its potential toxicity in pregnant women. Advise pregnant women of the potential harm to a fetus.
The estimated background risks of major birth defects and miscarriage for the indicated population(s) are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Vemurafenib showed no evidence of developmental toxicity in rat fetuses at doses up to 250 mg/kg/day (approximately 1.3 times the clinical exposure at 960 mg twice daily based on AUC) or rabbit fetuses at doses up to 450 mg/kg/day (approximately 0.6 times the clinical exposure at 960 mg twice daily based on AUC). Fetal drug levels were 3–5% of maternal levels, indicating that vemurafenib has the potential to be transmitted from the mother to the developing fetus.
8.2 Lactation
There is no information available regarding the presence of vemurafenib in human milk, effects on the breastfed infant, or effects on milk production. Because of the potential for serious adverse reactions in a breastfed infant, including malignancy, severe dermatologic reactions, QT prolongation, hepatotoxicity, photosensitivity, and ophthalmologic toxicity, [see Warnings and Precautions (5)], advise women not to breastfeed during treatment with ZELBORAF and for 2 weeks after the final dose.
8.3 Females and Males of Reproductive Potential
Contraception
Based on its mechanism of action, ZELBORAF can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with ZELBORAF and for 2 weeks after the final dose.
8.4 Pediatric Use
The safety and effectiveness of ZELBORAF in pediatric patients have not been established. Vemurafenib was studied in 6 adolescent patients 15 to 17 years of age with unresectable or metastatic melanoma with BRAF V600 mutation. A maximum tolerated dose was not reached with doses up to vemurafenib 960 mg twice daily. No new safety signals were observed. Vemurafenib steady-state exposure in these 6 adolescent patients was generally similar to that in adults.
8.5 Geriatric Use
Clinical studies of ZELBORAF did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
8.6 Hepatic Impairment
No formal clinical study has been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of vemurafenib. No dose adjustment is recommended for patients with mild and moderate hepatic impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology (12.3)]. The appropriate dose of ZELBORAF has not been established in patients with severe hepatic impairment.
Close8.7 Renal Impairment
No formal clinical study has been conducted to evaluate the effect of renal impairment on the pharmacokinetics of vemurafenib. No dose adjustment is recommended for patients with mild and moderate renal impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology (12.3)]. The appropriate dose of ZELBORAF has not been established in patients with severe renal impairment.
-
10 OVERDOSAGEThere is no information on overdosage of ZELBORAF.
-
11 DESCRIPTIONZELBORAF (vemurafenib) is a kinase inhibitor available as 240 mg tablets for oral use. Vemurafenib has the chemical name propane-1-sulfonic acid ...
ZELBORAF (vemurafenib) is a kinase inhibitor available as 240 mg tablets for oral use. Vemurafenib has the chemical name propane-1-sulfonic acid {3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]- 2,4-difluoro-phenyl}-amide. It has the molecular formula C23H18ClF2N3O3S and a molecular weight of 489.9. Vemurafenib has the following chemical structure:
Vemurafenib is a white to off-white crystalline solid. It is practically insoluble in aqueous media.
Tablets of ZELBORAF are for oral administration. Each tablet contains 240 mg of vemurafenib.
The inactive ingredients of ZELBORAF are: Tablet core: hypromellose acetate succinate, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, and hydroxypropyl cellulose. Coating: pinkish white: poly (vinyl alcohol), titanium dioxide, polyethylene glycol 3350, talc, and iron oxide red.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Vemurafenib is a low molecular weight, orally available inhibitor of some mutated forms of BRAF serine- threonine kinase, including BRAF V600E. Vemurafenib also ...
12.1 Mechanism of Action
Vemurafenib is a low molecular weight, orally available inhibitor of some mutated forms of BRAF serine- threonine kinase, including BRAF V600E. Vemurafenib also inhibits other kinases in vitro such as CRAF, ARAF, wild-type BRAF, SRMS, ACK1, MAP4K5, and FGR at similar concentrations. Some mutations in the BRAF gene including V600E result in constitutively activated BRAF proteins, which can cause cell proliferation in the absence of growth factors that would normally be required for proliferation. Vemurafenib has anti-tumor effects in cellular and animal models of melanomas with mutated BRAF V600E.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a multi-center, open-label, single-arm study in 132 patients with BRAF V600E mutation-positive metastatic melanoma, patients administered vemurafenib 960 mg orally twice daily did not experience large changes in mean QTc interval (i.e., > 20 ms) from baseline. Vemurafenib is associated with concentration- dependent QTc interval prolongation. The largest mean change from baseline in the first month of treatment occurred at 2 hours post-dose on Day 15—an increase of 12.8 ms (upper boundary of the two-sided 90% confidence interval of 14.9 ms). In the first 6 months of treatment, the largest observed mean change from baseline occurred at a pre-dose time point—an increase of 15.1 ms (upper boundary of the two-sided 90% confidence interval of 17.7 ms).
Close12.3 Pharmacokinetics
The pharmacokinetics of vemurafenib were determined in patients with BRAF mutation-positive metastatic melanoma following 15 days of 960 mg twice daily with dosing approximately 12 hours apart. The population pharmacokinetic analysis pooled data from 458 patients. At steady-state, vemurafenib exhibits linear pharmacokinetics within the 240 mg to 960 mg dose range.
Absorption
The mean bioavailability of vemurafenib at steady state was 64% (56% CV). The median time to reach maximum plasma vemurafenib concentration (Tmax) was 3 hours following multiple doses.
The mean (± SD) Cmax and AUC0-12 were 62 ± 17 µg/mL and 601 ± 170 µg*h/mL, respectively. The median accumulation ratio estimate from the population pharmacokinetic analysis for the twice daily regimen is 7.4, with steady-state achieved at approximately 15 to 22 days.
In clinical trials, vemurafenib was administered without regard to food. A food effect study has demonstrated that a single dose of vemurafenib administered with a high-fat meal increased AUC by approximately 5-fold, increased Cmax by 2.5-fold, and delayed Tmax by approximately 4 hours as compared to the fasted state.
QTc prolongation may occur with increased exposures as vemurafenib is associated with concentration-dependent QTc interval prolongation [see Clinical Pharmacology (12.2)].
Distribution
Vemurafenib is highly bound (> 99%) to human albumin and alpha-1 acid glycoprotein plasma proteins. The population apparent volume of distribution is estimated to be 106 L (with 66% inter-patient variability).
Metabolism
Following oral administration of 960 mg of 14C-vemurafenib, mean data showed that vemurafenib and its metabolites represented 95% and 5% of the components in plasma over 48 hours, respectively.
Elimination
Following oral administration of 960 mg of 14C-vemurafenib, approximately 94% of the radioactive dose was recovered in feces and approximately 1% was recovered in the urine. The population apparent clearance is estimated to be 31 L/day (with 32% inter-patient variability). The median elimination half-life estimate for vemurafenib is 57 hours (the 5th and 95th percentile range is 30 to 120 hours).
Specific Populations
Hepatic Impairment: The pharmacokinetics of vemurafenib were examined in patients with metastatic melanoma enrolled in the clinical trials with normal hepatic function (n=158, total bilirubin ≤ ULN) and mild (n=58, total bilirubin 1.0–1.5 × ULN), moderate (n=27, total bilirubin 1.5–3 × ULN), or severe (n=3, total bilirubin > 3 × ULN) hepatic impairment. Patients received vemurafenib 960 mg orally twice daily. The apparent clearance of vemurafenib in patients with mild and moderate hepatic impairment was similar to that in patients with normal hepatic function. The appropriate dose for patients with severe hepatic impairment cannot be determined as clinical and pharmacokinetic data were available for only three patients [see Use in Specific Populations (8.6)].
Renal Impairment: The pharmacokinetics of vemurafenib were examined in patients with metastatic melanoma enrolled in the clinical trials with normal renal function (CLcr ≥ 90 mL/min) and mild (n=94, CLcr > 60 to 89 mL/min), moderate (n=11, CLcr 30 to 59 mL/min) or severe (n=1, CLcr < 29 mL/min) renal impairment. Patients received vemurafenib 960 mg orally twice daily. The apparent clearance of vemurafenib in patients with mild and moderate renal impairment was similar to that in patients with normal renal function. The appropriate dose for patients with severe renal impairment cannot be determined as clinical and pharmacokinetic data were available for only one patient [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Effect of Strong CYP3A4 Inhibitors: Coadministration of 960 mg ZELBORAF twice daily with once daily doses of 200 mg itraconazole, a strong CYP3A4 inhibitor, increased steady state vemurafenib AUC0-τ by 40% (90% CI: 21%, 61%) with a similar magnitude of increase in Cmax [see Drug Interactions (7.1)].
Effect of Strong CYP3A4 Inducers: Coadministration of 600 mg daily doses of rifampin (a strong CYP3A inducer) with a single 960 mg dose of ZELBORAF decreased vemurafenib AUC by 40% (90% CI: 24%, 53%) with no effect on Cmax, relative to a 960 mg dose of ZELBORAF administered alone [see Dosage and Administration (2.4), Drug Interactions (7.1)].
Effect of Vemurafenib on CYP Substrates: In vitro studies suggest that vemurafenib is an inhibitor of CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4/5.
Coadministration of tizanidine 2 mg (a sensitive CYP1A2 substrate) on day 21 with vemurafenib which was administered 960 mg twice daily for 21 days increased tizanidine AUCinf by 4.7-fold (90% CI: 3.6, 6.3) and Cmax by 2.2-fold (90% CI: 1.7, 2.7) in 16 cancer patients [see Drug Interactions (7.2)]. In an in vivo phenotypic cocktail drug-drug interaction study in patients with cancer, a single dose of the CYP probe substrate cocktail (for CYP1A2, 2D6, 3A4, 2C19 and 2C9) was administered before and concomitantly with vemurafenib (following 15 days of dosing at 960 mg twice daily). Coadministration of vemurafenib increased the mean AUC of caffeine (CYP1A2 substrate) by 2.6-fold [see Drug Interactions (7.2)]. Coadministration of vemurafenib increased the mean AUC of dextromethorphan (CYP2D6 substrate) by 47% and the AUC of S-warfarin (CYP2C9 substrate) by 18%, while it decreased the mean AUC of midazolam (CYP3A4 substrate) by 39%. Coadministration of vemurafenib did not change the mean systemic exposure to omeprazole (CYP2C19 substrate).
Effect of Vemurafenib on Transporters: In vitro studies suggest that vemurafenib is both a substrate and an inhibitor of the efflux transporters P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP).
Administration of vemurafenib 960 mg twice daily for 22 days increased digoxin AUC by 1.8-fold (90% CI:1.6, 2.0) and Cmax by 1.5-fold (90% CI:1.3, 1.7) in 26 cancer patients who were coadministered a single dose of digoxin 0.25 mg (sensitive P-gp substrate) [see Drug Interactions (7.4)].
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There have been no formal studies conducted assessing the carcinogenic potential of vemurafenib. ZELBORAF increased the development of ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no formal studies conducted assessing the carcinogenic potential of vemurafenib. ZELBORAF increased the development of cutaneous squamous cell carcinomas in patients in clinical trials.
Vemurafenib did not cause genetic damage when tested in in vitro assays (bacterial mutation [AMES Assay], human lymphocyte chromosome aberration) or in the in vivo rat bone marrow micronucleus test.
No specific studies with vemurafenib have been conducted in animals to evaluate the effect on fertility; nevertheless, no histopathological findings were noted in reproductive organs in males and females in repeat-dose toxicology studies in rats at doses up to 450 mg/kg/day (approximately 0.6 and 1.6 times the human exposure based on AUC in males and females, respectively) and dogs at doses up to 450 mg/kg/day (approximately 0.3 times the human clinical exposure based on AUC in both males and females, respectively).
Close13.2 Animal Toxicology and/or Pharmacology
Consistent with the increased incidence of cutaneous squamous cell carcinomas in patients treated with vemurafenib, the treatment of mice implanted with human cuSCC cells with vemurafenib caused a dose- dependent acceleration of the growth of the implanted tumors.
-
14 CLINICAL STUDIESTreatment-Naïve Patients with BRAF V600E Mutation-Positive Unresectable or Metastatic Melanoma - Trial 1, an international, open-label, randomized controlled trial, equally allocated 675 ...
Treatment-Naïve Patients with BRAF V600E Mutation-Positive Unresectable or Metastatic Melanoma
Trial 1, an international, open-label, randomized controlled trial, equally allocated 675 patients with treatment-naive, BRAF V600E mutation-positive unresectable or metastatic melanoma, as detected by the cobas® 4800 BRAF V600 Mutation Test, to receive ZELBORAF 960 mg by mouth twice daily (n=337) or dacarbazine 1000 mg/m2 intravenously on Day 1 every 3 weeks (n=338). Randomization stratification factors were disease stage, lactate dehydrogenase (LDH), ECOG performance status, and geographic region. Treatment continued until disease progression, unacceptable toxicity, and/or consent withdrawal. The major efficacy outcome measures of the trial were overall survival (OS) and investigator-assessed progression-free survival (PFS). Other outcome measures included confirmed investigator-assessed best overall response rate.
Baseline characteristics were balanced between treatment groups. Most patients were male (56%) and Caucasian (99%), the median age was 54 years (24% were ≥ 65 years), all patients had ECOG performance status of 0 or 1, and the majority of patients had metastatic disease (95%).
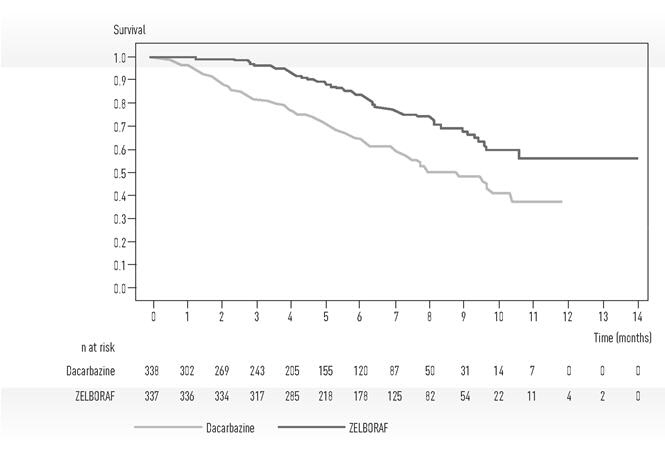
Trial 1 demonstrated statistically significant increases in overall survival and progression-free survival in the ZELBORAF arm compared to the dacarbazine control arm. Table 5 and Figure 1 summarize the efficacy results.
Table 5 Efficacy of ZELBORAF in Treatment-Naïve Patients with BRAF V600E Mutation-Positive Melanoma* ZELBORAF
(n=337)Dacarbazine
(n=338)p-value† - *
- As detected by the cobas® 4800 BRAF V600 Mutation Test
- †
- Unstratified log-rank test
- ‡
- Total of 200 deaths (ZELBORAF median follow-up 6.2 months)
- §
- Hazard ratio estimated using Cox model; a hazard ratio of < 1 favors ZELBORAF
- ¶
- Kaplan-Meier estimate
- #
- Updated based on 478 deaths (ZELBORAF median follow-up 13.4 months)
Overall Survival Number of Deaths‡ 78 (23%) 122 (36%) Hazard Ratio
(95% CI)§0.47
(0.35, 0.62)< 0.0001 Updated Median Survival (months)
(95 % CI) ¶, #13.6
(12.0, 15.3)10.3
(9.1, 12.8)- Progression-Free Survival Hazard Ratio
(95% CI)§0.26
(0.20, 0.33)< 0.0001 Median PFS (months)
(95% CI)¶5.3
(4.9, 6.6)1.6
(1.6, 1.7)- Figure 1 Kaplan-Meier Curves of Overall Survival – Treatment-Naïve Patients
The confirmed, investigator-assessed best overall response rate was 48.4% (95% CI: 41.6%, 55.2%) in the ZELBORAF arm compared to 5.5% (95% CI: 2.8%, 9.3%) in the dacarbazine arm. There were 2 complete responses (0.9%) and 104 partial responses (47.4%) in the ZELBORAF arm and all 12 responses were partial responses (5.5%) in the dacarbazine arm.
Patients with BRAF V600E Mutation-Positive Metastatic Melanoma Who Received Prior Systemic Therapy
In a single-arm, multicenter, multinational trial (Trial 2), 132 patients with BRAF V600E mutation-positive metastatic melanoma, as detected by the cobas® 4800 BRAF V600 Mutation Test, who had received at least one prior systemic therapy, received ZELBORAF 960 mg by mouth twice daily. The median age was 52 years with 19% of patients being older than 65 years. The majority of patients were male (61%) and Caucasian (99%). Forty-nine percent of patients received ≥ 2 prior therapies. The median duration of follow-up was 6.87 months (range, 0.6 to 11.3).
The confirmed best overall response rate as assessed by an independent review committee (IRC) was 52% (95% CI: 43%, 61%). There were 3 complete responses (2.3%) and 66 partial responses (50.0%). The median time to response was 1.4 months with 75% of responses occurring by month 1.6 of treatment. The median duration of response by IRC was 6.5 months (95% CI: 5.6, not reached).
Patients with BRAF V600E Mutation-Positive Melanoma with Brain Metastases
The activity of ZELBORAF for the treatment of BRAF V600E mutation-positive melanoma, metastatic to the brain was evaluated in an open-label, multicenter, single-arm, two cohort trial (Trial 3). All patients received ZELBORAF 960 mg orally twice daily until disease progression or unacceptable toxicity. Patients were required to have at least one measurable brain lesion of 0.5 cm or greater on contrast-enhanced MRI, a stable or decreasing corticosteroid dose and no prior treatment with a BRAF or MEK inhibitor. Patients in Cohort A had received no prior local therapy for brain metastases. Patients in Cohort B had received at least one prior local therapy for brain metastases (surgical resection, whole brain radiotherapy, or stereotactic radiotherapy) with CNS progression following this therapy. Patients were followed until death, disease progression, withdrawal, or up to 24 months. The primary efficacy outcome measure was the confirmed best overall response rate in the brain in Cohort A, as assessed by an independent radiology review committee using Response Evaluation Criteria in Solid Tumors (RECIST v1.1). Secondary efficacy outcome measures included duration of response in Cohort A, and confirmed best overall response rate and duration of response in Cohort B.
A total of 146 patients (Cohort A: n=90; Cohort B: n=56) were enrolled and received at least one dose of ZELBORAF. In Cohort A, the median age of patients was 56 years, 62% were male, 47% had a pre- treatment ECOG performance status (PS) of 0, 57% had an elevated LDH value at baseline, and 20% received one or more systemic regimens for the treatment of metastatic disease. In Cohort B, the median age of patients was 53 years, 61% were male, 38% had a pre-treatment ECOG PS of 0, 55% had an elevated LDH value at baseline, and 39% received one or more systemic regimens for the treatment of metastatic disease. All patients enrolled on Trial 3 whose race was identified were White. The efficacy results are summarized in Table 6.
Table 6 Efficacy Results in Patients with BRAF V600E Melanoma Brain Metastases Cohort A
(n=90)Cohort B
(n=56)Confirmed Best Overall Response Rate in Brain, 95%CI* 18% (11%, 27%) 18% (9%, 30%) Complete response 2% 0 Partial response 16% 18% Median of Duration of Response, months (95%CI†) 4.6 (2.9, 6.2) 6.6 (2.8, 10.7) Patients with Wild-Type BRAF Melanoma
ZELBORAF has not been studied in patients with wild-type BRAF melanoma [see Warnings and Precautions (5.2)].
ClosePatients with Erdheim-Chester Disease (ECD)
An open-label, multicenter, single-arm, multiple cohort study of ZELBORAF (Trial 4) was conducted in patients ≥ 16 years of age with non-melanoma BRAF V600 mutation–positive diseases.
The trial included 22 patients with ECD. Fifteen patients (68.2%) had received prior systemic therapies. The median age was 58.5 years (range, 34 to 77 years). Fifty-five percent of patients were men.
All 22 patients received a starting dose of 960 mg orally twice daily with or without food. For 8 patients, the dose was reduced to 720 mg twice daily. For the remaining 14 patients, the dose was ultimately reduced to 480 mg. The median duration of treatment following a dose reduction to 720 mg was 77 days (range, 4 to 1325) and to 480 mg was 236 days (range, 21 to 924). The efficacy was maintained in these patients based on the overall response rate.
The efficacy of ZELBORAF in ECD was based on best overall response rate maintained on two occasions at least four weeks apart, as assessed by the investigator using RECIST v 1.1, and is presented in Table 7 below. The median duration of follow up was 26.6 months in ECD patients (range, 3.0 to 44.3 months). The median time to response was 11 months (95% CI: 3.7, 14.6). The median DOR was not estimable.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZELBORAF (vemurafenib) is supplied as 240 mg film-coated tablets with VEM debossed on one side. The following packaging configurations are available: NDC 50242-090-01 single bottle of 120 ...
ZELBORAF (vemurafenib) is supplied as 240 mg film-coated tablets with VEM debossed on one side. The following packaging configurations are available:
NDC 50242-090-01 single bottle of 120 count
NDC 50242-090-02 single bottle of 112 count
Storage and Stability: Store at room temperature 20°C–25°C (68°F–77°F); excursions permitted between 15°C and 30°C (59°F and 86°F), See USP Controlled Room Temperature. Store in the original container with the lid tightly closed.
CloseDisposal of unused/expired medicines: The release of pharmaceuticals in the environment should be minimized. Medicines should not be disposed of via wastewater and disposal through household waste should be avoided. Use established "collection systems," if available in your location.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Healthcare providers should advise patients of the potential benefits and risks of ZELBORAF and instruct their ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Healthcare providers should advise patients of the potential benefits and risks of ZELBORAF and instruct their patients to read the Medication Guide before starting ZELBORAF therapy. Inform patients of the following:
- Evidence of BRAF V600E mutation in the tumor specimen with an FDA approved test is necessary to identify patients with melanoma for whom treatment with ZELBORAF is indicated [see Dosage and Administration (2.1)].
- ZELBORAF increases the risk of developing new primary cutaneous malignancies. Advise patients of the importance of contacting their healthcare provider immediately for any changes in their skin [see Warnings and Precautions (5.1)].
- Anaphylaxis and other serious hypersensitivity reactions can occur during treatment and upon re- initiation of treatment with ZELBORAF. Advise patients to stop taking ZELBORAF and to seek immediate medical attention for symptoms of anaphylaxis or hypersensitivity [see Warnings and Precautions (5.3)].
- Severe dermatologic reactions can occur in patients receiving ZELBORAF. Advise patients to stop taking ZELBORAF and to contact their health-care provider for severe dermatologic reactions [see Warnings and Precautions (5.4)].
- ZELBORAF can prolong QT interval, which may result in ventricular arrhythmias. Advise patients of the importance of monitoring of their electrolytes and the electrical activity of their heart (via an ECG) during ZELBORAF treatment [see Warnings and Precautions (5.5)].
- Liver injury leading to functional hepatic impairment, including coagulopathy or other organ dysfunction, can occur with ZELBORAF. Advise patients of the importance of laboratory monitoring of their liver during ZELBORAF treatment and to contact their health-care provider for relevant symptoms [see Warnings and Precautions (5.6)].
- ZELBORAF can cause mild to severe photosensitivity. Advise patients to avoid sun exposure, wear protective clothing, and use a broad spectrum UVA/UVB sunscreen and lip balm (SPF ≥ 30) when outdoors to help protect against sunburn [see Warnings and Precautions (5.7)].
- Ophthalmologic reactions can occur in patients treated with ZELBORAF. Advise patients to contact their health-care provider immediately for ophthalmologic symptoms [see Warnings and Precautions (5.8)].
Embryo-fetal Toxicity
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus [see Warnings and Precautions (5.9) and Use in Special Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with ZELBORAF and for 2 weeks after the final dose [see Warnings and Precautions (5.9) and Use in Special Populations (8.1, 8.3)].
- Advise female patients to contact their health-care provider immediately with a known or suspected pregnancy [see Warnings and Precautions (5.9) and Use in Special Populations (8.1, 8.3)].
CloseLactation
- Advise a woman not to breastfeed during treatment with ZELBORAF and for 2 weeks after the final dose [see Use in Specific Populations (8.2)].
- Radiation sensitization and recall can occur in patients treated with radiation prior to, during, or subsequent to ZELBORAF treatment. Advise patients to inform their health care provider if they have had or are planning to receive radiation therapy [see Warnings and Precautions (5.10), Adverse Reactions (6.2)].
- Renal failure can occur in patients treated with ZELBORAF. Advise patients of the importance of monitoring serum creatinine prior to and during ZELBORAF treatment [see Warnings and Precautions (5.11), Adverse Reactions (6.2)].
- Advise patients to contact their health care provider for symptoms of Dupuytren's contracture or plantar fascial fibromatosis [see Warnings and Precautions (5.12)].
-
SPL UNCLASSIFIED SECTIONDistributed by: Genentech USA, Inc. A Member of the Roche Group - 1 DNA Way - South San Francisco, CA 94080-4990 - ZELBORAF is a registered trademark of Genentech, Inc. Co-promoted by: Genentech USA ...
Distributed by:
Genentech USA, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990ZELBORAF is a registered trademark of Genentech, Inc.
Close
Co-promoted by: Genentech USA, Inc. and Daiichi Sankyo, Inc.
©2020 Genentech, Inc. -
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug AdministrationRevised: 11/2017 - MEDICATION GUIDE - ZELBORAF® (ZEL-bor-raf) (vemurafenib) tablet - What is the most ...Close
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 11/2017 MEDICATION GUIDE
ZELBORAF® (ZEL-bor-raf)
(vemurafenib)
tabletWhat is the most important information I should know about ZELBORAF?
ZELBORAF can cause serious side effects, including:
Risk of new cancers. ZELBORAF may cause certain types of skin cancer called cutaneous squamous cell carcinoma (cuSCC) and keratoacanthoma. New melanoma lesions have occurred in people who take ZELBORAF. ZELBORAF may also cause another type of cancer called non-cutaneous squamous cell carcinoma (non-cuSCC). Talk with your healthcare provider about your risk for these cancers.
Check your skin and tell your healthcare provider right away about any skin changes including a:
- new wart
- skin sore or reddish bump that bleeds or does not heal
- change in size or color of a mole
Your healthcare provider should check your skin before you start taking ZELBORAF, and every 2 months during treatment with ZELBORAF, to look for any new skin cancers. Your healthcare provider may continue to check your skin for 6 months after you stop taking ZELBORAF.
Your healthcare provider should also check for cancers that may not occur on the skin. Tell your healthcare provider about any new symptoms that you get while taking ZELBORAF.
Other blood cell cancers have happened in some people with Erdheim-Chester Disease (ECD) including those who take ZELBORAF. If you have other blood cell cancers and take ZELBORAF for ECD, your healthcare provider will monitor your blood cancer through routine blood tests.
See "What are the possible side effects of ZELBORAF?" for more information about side effects.
What is ZELBORAF?
ZELBORAF is a prescription medicine used to treat:
- a type of skin cancer called melanoma that:
- has spread to other parts of the body or cannot be removed by surgery, and
- has a certain type of abnormal "BRAF" gene.
ZELBORAF is not used to treat melanoma with a normal BRAF gene.
Your healthcare provider will perform a test to make sure that ZELBORAF is right for you.
- a type of blood cell cancer called Erdheim-Chester Disease (ECD) that:
- can affect body tissues and organs, and
- has a certain type of abnormal "BRAF" gene.
It is not known if ZELBORAF is safe and effective in children under 18 years of age.
Before you take ZELBORAF, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called long QT syndromes
- have liver or kidney problems
- have had or are planning to receive radiation therapy
- have been told that you have low blood levels of potassium, calcium, or magnesium
- are pregnant or plan to become pregnant. ZELBORAF can harm your unborn baby.
- Females who are able to become pregnant should use effective birth control during treatment with ZELBORAF and for 2 weeks after the final dose of ZELBORAF.
- Talk to your healthcare provider about birth control methods that may be right for you.
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with ZELBORAF.
- are breastfeeding or plan to breastfeed. It is not known if ZELBORAF passes into your breast milk. Do not breastfeed during treatment with ZELBORAF and for 2 weeks after the final dose of ZELBORAF. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ZELBORAF?
- Take ZELBORAF exactly as your healthcare provider tells you. Do not change your dose or stop taking ZELBORAF unless your healthcare provider tells you to.
- Take ZELBORAF every 12 hours with or without a meal.
- Do not crush or chew ZELBORAF tablets.
- Do not take an additional dose of ZELBORAF if you vomit after taking your scheduled dose. Take your next dose at your regular time.
- If you miss a dose of ZELBORAF, take it as soon as you remember. If it is within 4 hours of your next scheduled dose, just take your next dose at your regular time. Do not make up for the missed dose.
- If you take too much ZELBORAF, call your healthcare provider or go the nearest hospital emergency room right away.
What should I avoid while taking ZELBORAF?
Avoid sunlight during treatment with ZELBORAF. ZELBORAF can make your skin sensitive to sunlight. You may burn more easily and get severe sunburns. To help protect against sunburn:
- When you go outside, wear clothes that protect your skin, including your head, face, hands, arms, and legs.
- Use lip balm and a broad-spectrum sunscreen with SPF 30 or higher.
What are the possible side effects of ZELBORAF?
ZELBORAF may cause serious side effects, including:
- See "What is the most important information I should know about ZELBORAF?"
- Allergic reactions can happen while taking ZELBORAF and can be severe. Stop taking ZELBORAF and get medical help right away if you get any of these symptoms of an allergic reaction:
- rash or redness all over your body
- trouble breathing or swallowing
- swelling of the face, lips, or tongue
- throat tightness or hoarseness
- feel faint
- a fast heartbeat
- blisters on your skin
- blisters or sores in your mouth
- peeling of your skin
- fever
- redness or swelling of your face, hands, or soles of your feet
-
Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider should do tests before you start taking ZELBORAF and during your treatment with ZELBORAF to check the electrical activity of your heart and your body salts (electrolytes). Tell your healthcare provider right away if you feel faint, lightheaded, dizzy, or feel your heart beating irregularly or fast while taking ZELBORAF. These may be symptoms related to QT prolongation.
-
Liver injury. Your healthcare provider should do blood tests to check your liver function before you start taking ZELBORAF and during treatment. Tell your healthcare provider right away if you get any of these symptoms of a liver problem during treatment:
- yellowing of your skin or the white part of your eyes
- dark or brown (tea color) urine
- nausea or vomiting
- loss of appetite
- pain on the right side of your stomach
-
Eye problems. Tell your healthcare provider right away if you get any of these symptoms during treatment with ZELBORAF:
- eye pain, swelling, or redness
- blurred vision or other vision changes
- Worsening side effects from radiation treatment that can sometimes be severe or lead to death. Tell your healthcare provider if you have had or are planning to receive radiation therapy.
- Kidney injury. Your healthcare provider should do blood tests to check your kidney function before you start taking ZELBORAF and during treatment.
- Connective tissue disorders. Tell your healthcare provider if you develop an unusual thickening of the palms of your hands along with tightening of the fingers inward or any unusual thickening of the soles of your feet which may be painful.
The most common side effects of ZELBORAF in melanoma include:
- joint pain
- rash (see "Severe skin reactions" above)
- hair loss
- tiredness
- □
- sunburn or sun sensitivity
- □
- nausea
- □
- itching
- □
- warts
The most common side effects of ZELBORAF in Erdheim-Chester Disease include:
- joint pain
- rash
- warts
- □
- tiredness
- □
- hair loss
- QT prolongation (see "Changes in the electrical activity of your heart called QT prolongation" above)
These are not all the possible side effects of ZELBORAF.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Genentech at 1-888-835-2555.
How should I store ZELBORAF?
- Store ZELBORAF at room temperature between 68°F to 77°F (20°C to 25°C).
- Store ZELBORAF in the original container with the lid tightly closed.
- Ask your healthcare provider or pharmacist how to safely throw away (dispose of) any unused or expired ZELBORAF.
Keep ZELBORAF and all medicine out of the reach of children.
General information about the safe and effective use of ZELBORAF.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZELBORAF for a condition for which it was not prescribed. Do not give ZELBORAF to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ZELBORAF that is written for health professionals.
What are the ingredients in ZELBORAF?
Active ingredient: vemurafenib
Inactive ingredients:
Tablet Core: hypromellose acetate succinate, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, and hydroxypropyl cellulose.
Coating: pinkish white: poly (vinyl alcohol), titanium dioxide, polyethylene glycol 3350, talc, and iron oxide red.
Distributed by: Genentech USA, Inc., A Member of the Roche Group,1 DNA Way, South San Francisco, CA 94080-4990
ZELBORAF is a registered trademark of Genentech, Inc.
Co-promoted by: Genentech USA, Inc. and Daiichi Sankyo, Inc.
©2017 Genentech, Inc. For more information, go to www.ZELBORAF.com or call 1-877-436-3683. -
SPL UNCLASSIFIED SECTIONRepresentative sample of labeling (see the HOW SUPPLIED section for complete listing):
-
PRINCIPAL DISPLAY PANEL - 240 mg Tablet Bottle CartonNDC 50242-090-02 - Zelboraf® (vemurafenib) tablets - 240 mg - Do not crush or chew tablet. Rx only - Attention Pharmacist: Dispense the - accompanying Medication Guide to - each patient. 112 ...
NDC 50242-090-02
Zelboraf®
(vemurafenib)
tablets240 mg
Do not crush or chew tablet.
Rx only
Attention Pharmacist: Dispense the
accompanying Medication Guide to
each patient.112 tablets
Genentech | Daiichi Sankyo, Inc.
10210025
Close
-
INGREDIENTS AND APPEARANCEProduct Information
ZELBORAF vemurafenib tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-090 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VEMURAFENIB (UNII: 207SMY3FQT) (VEMURAFENIB - UNII:207SMY3FQT) VEMURAFENIB 240 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE ACETATE SUCCINATE 16070722 (3 MPA.S) (UNII: 24P2YXD2PW) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE (90000 WAMW) (UNII: UKE75GEA7F) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color ORANGE, WHITE (Pinkish white to orange white) Score no score Shape OVAL (biconvex) Size 19mm Flavor Imprint Code VEM Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-090-02 1 in 1 CARTON 09/08/2015 1 112 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:50242-090-86 1 in 1 CARTON 04/10/2014 03/31/2022 2 112 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202429 08/17/2011 Labeler - Genentech, Inc. (080129000)
CloseEstablishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche AG 482242971 API MANUFACTURE(50242-090) , ANALYSIS(50242-090) , MANUFACTURE(50242-090)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ZELBORAF- vemurafenib tablet, film coated
Number of versions: 23
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Nov 25, 2024 | 26 (current) | download |
| Jul 11, 2024 | 25 | download |
| Dec 1, 2023 | 24 | download |
| Apr 6, 2023 | 23 | download |
| Oct 28, 2021 | 22 | download |
| Nov 5, 2020 | 21 | download |
| May 28, 2020 | 20 | download |
| Dec 20, 2018 | 19 | download |
| Dec 28, 2017 | 18 | download |
| Nov 16, 2017 | 17 | download |
| Sep 22, 2017 | 16 | download |
| Apr 24, 2017 | 15 | download |
| Sep 13, 2016 | 14 | download |
| May 23, 2016 | 13 | download |
| Aug 24, 2015 | 12 | download |
| Feb 2, 2015 | 11 | download |
| Jan 7, 2015 | 10 | download |
| Apr 1, 2014 | 8 | download |
| Feb 24, 2014 | 7 | download |
| Aug 16, 2013 | 6 | download |
| Jul 23, 2013 | 5 | download |
| Apr 16, 2013 | 4 | download |
| Aug 19, 2011 | 2 | download |
RxNorm
ZELBORAF- vemurafenib tablet, film coated
Get Label RSS Feed for this Drug
ZELBORAF- vemurafenib tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=38eea320-7e0c-485a-bc30-98c3c45e2763
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ZELBORAF- vemurafenib tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50242-090-02 |
| 2 | 50242-090-86 |

