Label: ZAVESCA- miglustat capsule
- NDC Code(s): 66215-201-15, 66215-201-90
- Packager: Actelion Pharmaceuticals US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZAVESCA safely and effectively. See full prescribing information for ZAVESCA. ZAVESCA - ® (miglustat) capsules, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Type 1 Gaucher Disease - ZAVESCA is indicated as monotherapy for the treatment of adult patients with mild to moderate type 1 Gaucher disease for whom enzyme replacement therapy is not a ...
-
2 DOSAGE AND ADMINISTRATION2.1 Instructions for Administration - Therapy should be directed by physicians who are knowledgeable in the management of Gaucher disease. The recommended dose for the treatment of adult patients ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 100 mg of miglustat, white opaque hard gelatin capsules with "OGT 918" printed in black on the cap and "100" printed in black on the body.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Peripheral Neuropathy - In clinical trials, cases of peripheral neuropathy have been reported in 3% of Gaucher's patients treated with ZAVESCA. All patients receiving ZAVESCA treatment should ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Peripheral Neuropathy - [see - Warnings and Precautions (5.1) ...
-
7 DRUG INTERACTIONSWhile co-administration of ZAVESCA appeared to increase the clearance of imiglucerase by 70%, these results are not conclusive because of the small number of patients studied and because patients ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal reproduction studies, ZAVESCA may cause fetal harm when administered to a pregnant woman. Available data from postmarketing case ...
-
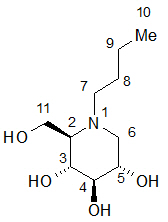
11 DESCRIPTIONZAVESCA - ® (miglustat capsules, 100 mg) is a glucosylceramide synthase inhibitor, which is a glucosyl transferase enzyme responsible for the first step in the synthesis of most ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Type 1 Gaucher disease is caused by a functional deficiency of glucocerebrosidase, the enzyme that mediates the degradation of the glycosphingolipid ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Two-year carcinogenicity studies have been conducted with miglustat in CD-1 mice at oral doses up to 500 mg/kg/day ...
-
14 CLINICAL STUDIESThe efficacy of ZAVESCA in type 1 Gaucher disease has been investigated in two open-label, uncontrolled trials and one randomized, open-label, active-controlled trial with enzyme replacement given ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZAVESCA is supplied in hard gelatin capsules containing 100 mg of miglustat. ZAVESCA - ® 100 mg capsules are white opaque with "OGT 918" printed in black on the cap and "100" printed in ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Information for Patients - Advise patients that the most common serious adverse reaction reported with ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company - Titusville, NJ 08560, USA - © 2003 – 2017 Actelion Pharmaceuticals US ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - ZAVESCA - ® (zah-VEHS-kah) (miglustat) capsules - This ...
-
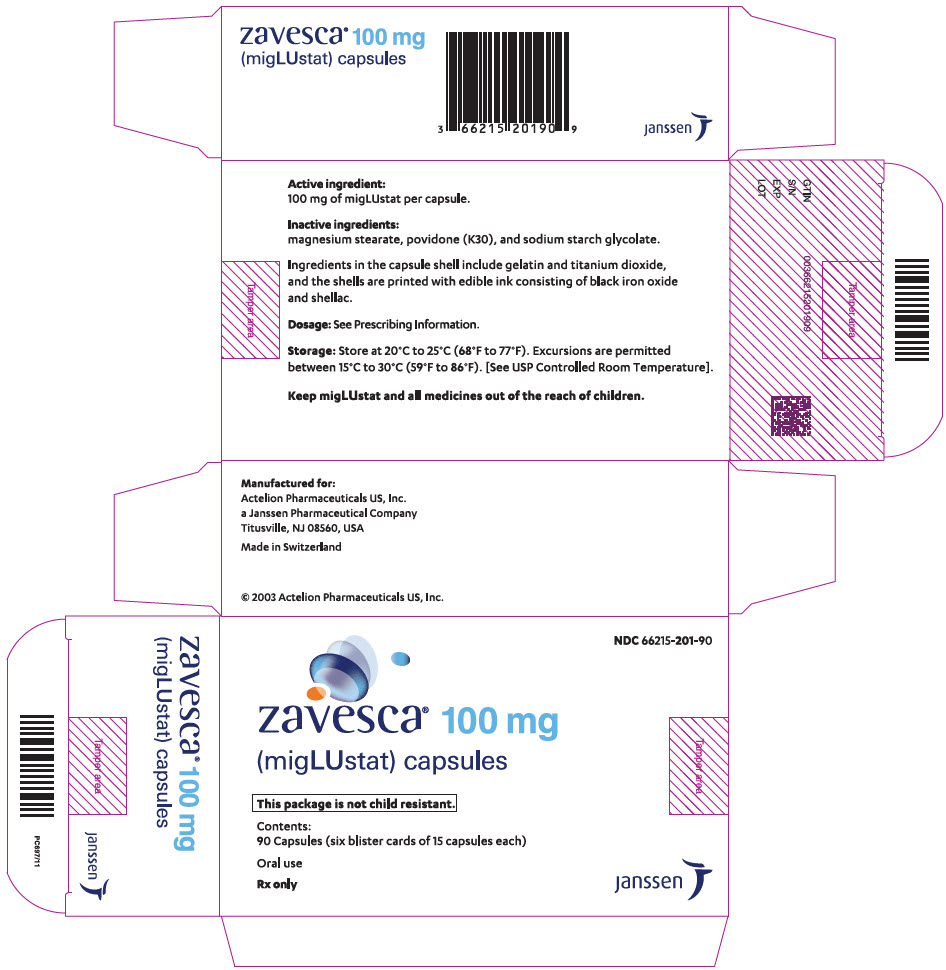
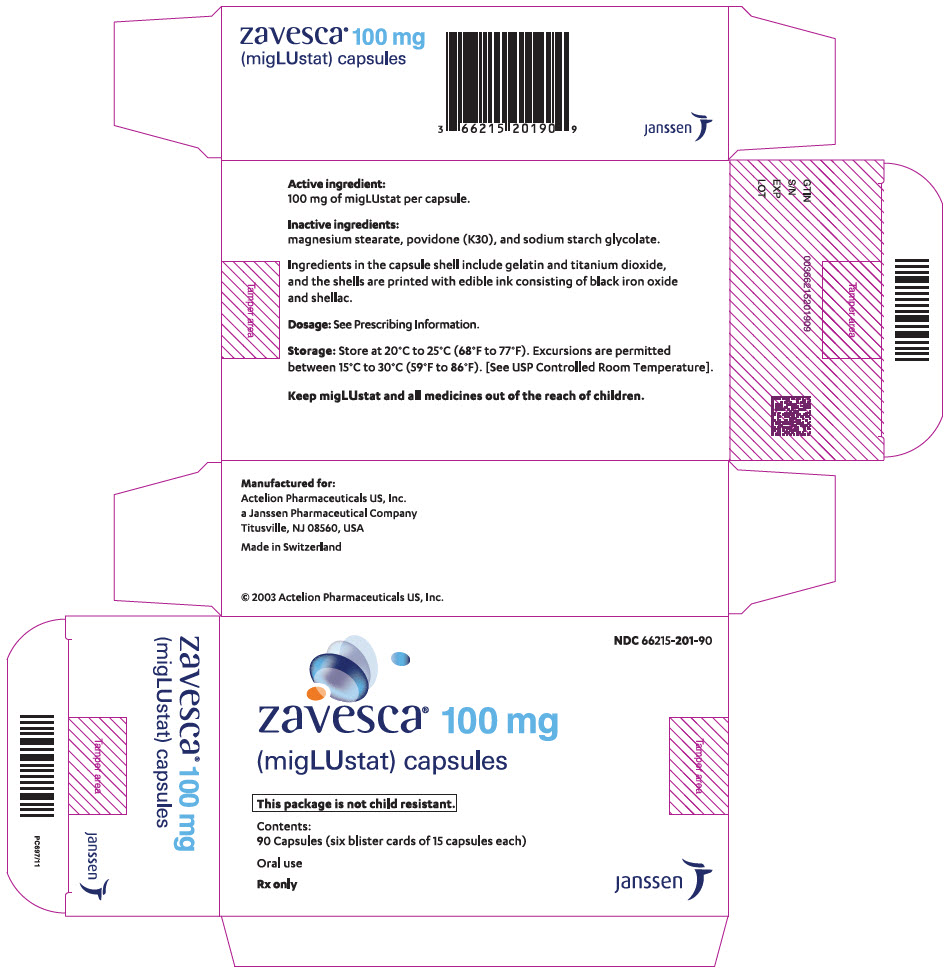
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Blister Card CartonNDC 66215-201-90 - zavesca - ® 100 mg - (migLUstat) capsules - This package is not child resistant. Contents: 90 Capsules (six blister cards of 15 ...
-
INGREDIENTS AND APPEARANCEProduct Information