Label: ZALTRAP- ziv-aflibercept solution, concentrate
- NDC Code(s): 0024-5840-01, 0024-5840-03, 0024-5841-01
- Packager: sanofi-aventis U.S. LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZALTRAP safely and effectively. See full prescribing information for ZALTRAP. ZALTRAP® (ziv-aflibercept) injection, for intravenous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZALTRAP, in combination with fluorouracil, leucovorin, irinotecan-(FOLFIRI), is indicated for the treatment of patients with metastatic colorectal cancer (mCRC) that is resistant to or has ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose and Schedule - The recommended dosage of ZALTRAP is 4 mg per kg of actual body weight as an intravenous infusion over 1 hour every two weeks in combination with FOLFIRI until ...
-
3 DOSAGE FORMS AND STRENGTHSZALTRAP is a clear, colorless to pale-yellow solution available as: Injection: 100 mg/4 mL (25 mg/mL) solution in a single-dose vial - Injection: 200 mg/8 mL (25 mg/mL) solution in a single-dose ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hemorrhage - Patients treated with ZALTRAP have an increased risk of hemorrhage, including severe and sometimes fatal hemorrhagic events. In patients with mCRC, bleeding/hemorrhage (all ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hemorrhage [see Warnings and Precautions (5.1)] Gastrointestinal Perforation [see Warnings and ...
-
7 DRUG INTERACTIONSNo dedicated drug-drug interaction studies have been conducted for ZALTRAP. No clinically important pharmacokinetic interactions were found between ziv-aflibercept and irinotecan/SN-38 or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal reproduction studies and its mechanism of action [see Clinical Pharmacology (12.1)], ZALTRAP can cause fetal harm when administered ...
-
11 DESCRIPTIONZiv-aflibercept is a vascular endothelial growth factor inhibitor. It is a recombinant fusion protein consisting of Vascular Endothelial Growth Factor (VEGF)-binding portions from the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ziv-aflibercept acts as a soluble receptor that binds to human VEGF-A (equilibrium dissociation constant KD of 0.5 pM for VEGF-A165 and 0.36 pM for VEGF-A121), to human ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted to evaluate carcinogenicity or mutagenicity of ziv-aflibercept. Ziv-aflibercept impaired reproductive ...
-
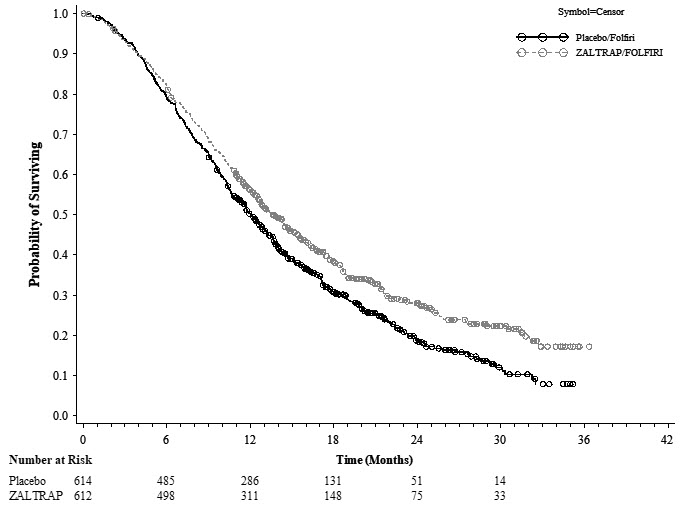
14 CLINICAL STUDIESThe efficacy of ZALTRAP was evaluated in VELOUR (NCT00561470), a randomized (1:1), double-blind, placebo-controlled study in patients with mCRC who are resistant to or have progressed during or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZALTRAP (ziv-aflibercept) injection is a clear, colorless to pale-yellow solution supplied in single-dose vials with a concentration of 25 mg/mL. NDC 0024-5840-01: carton containing one ...
-
17 PATIENT COUNSELING INFORMATIONHemorrhage - Inform patients that ZALTRAP can cause severe bleeding and advise patients to contact their healthcare provider for bleeding or symptoms of bleeding, including lightheadedness ...
-
SPL UNCLASSIFIED SECTIONManufactured by: sanofi-aventis U.S. LLC - Bridgewater, NJ 08807 - A SANOFI COMPANY - U.S. License No. 1752 - ZALTRAP is a registered trademark of Regeneron Pharmaceuticals, Inc. ©2023 sanofi-aventis U.S ...
-
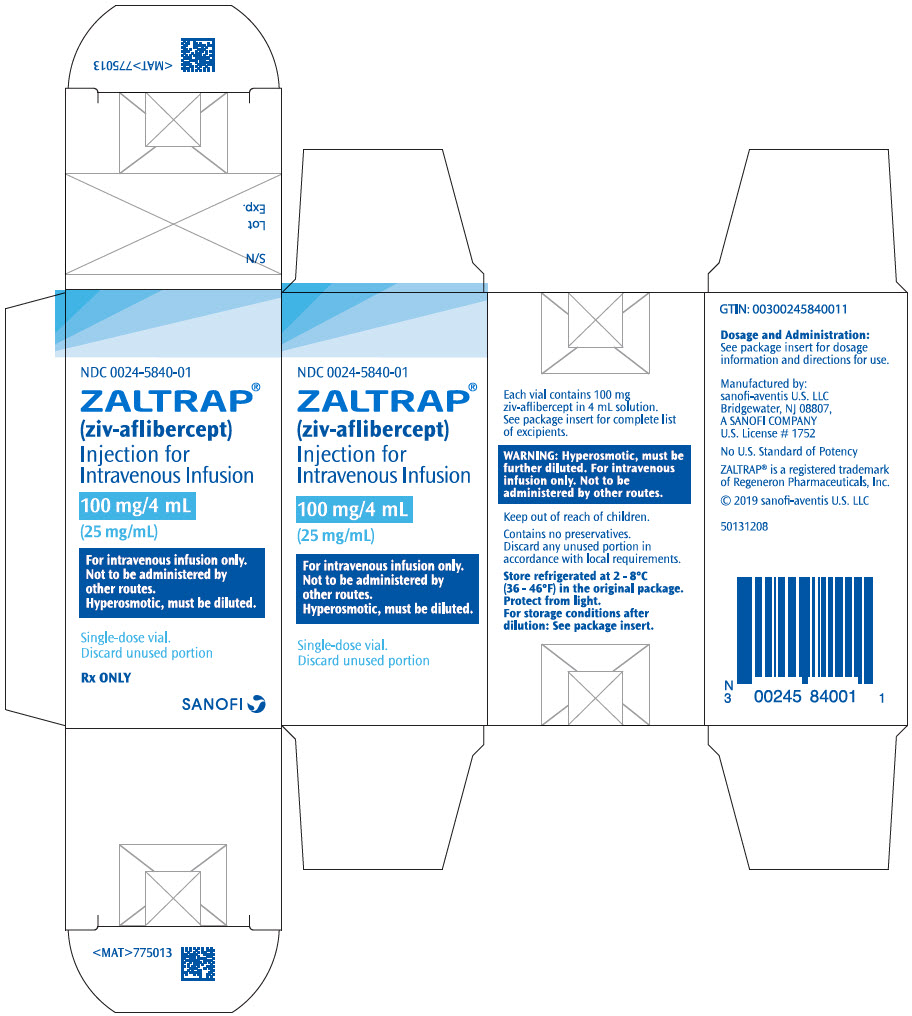
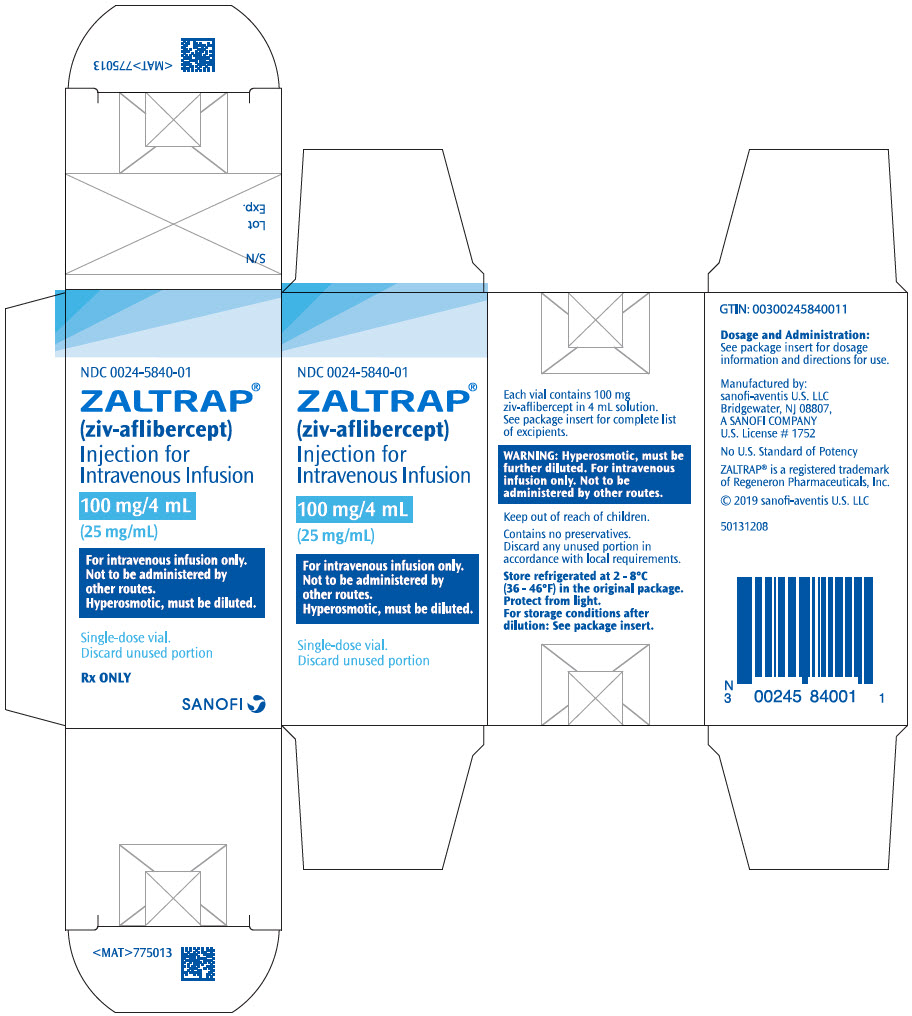
PRINCIPAL DISPLAY PANEL - 100 mg/4 mL Vial CartonNDC 0024-5840-01 - ZALTRAP® (ziv-aflibercept) Injection for - Intravenous Infusion - 100 mg/4 mL - (25 mg/mL) For intravenous infusion only. Not to be administered by - other routes. Hyperosmotic, must be ...
-
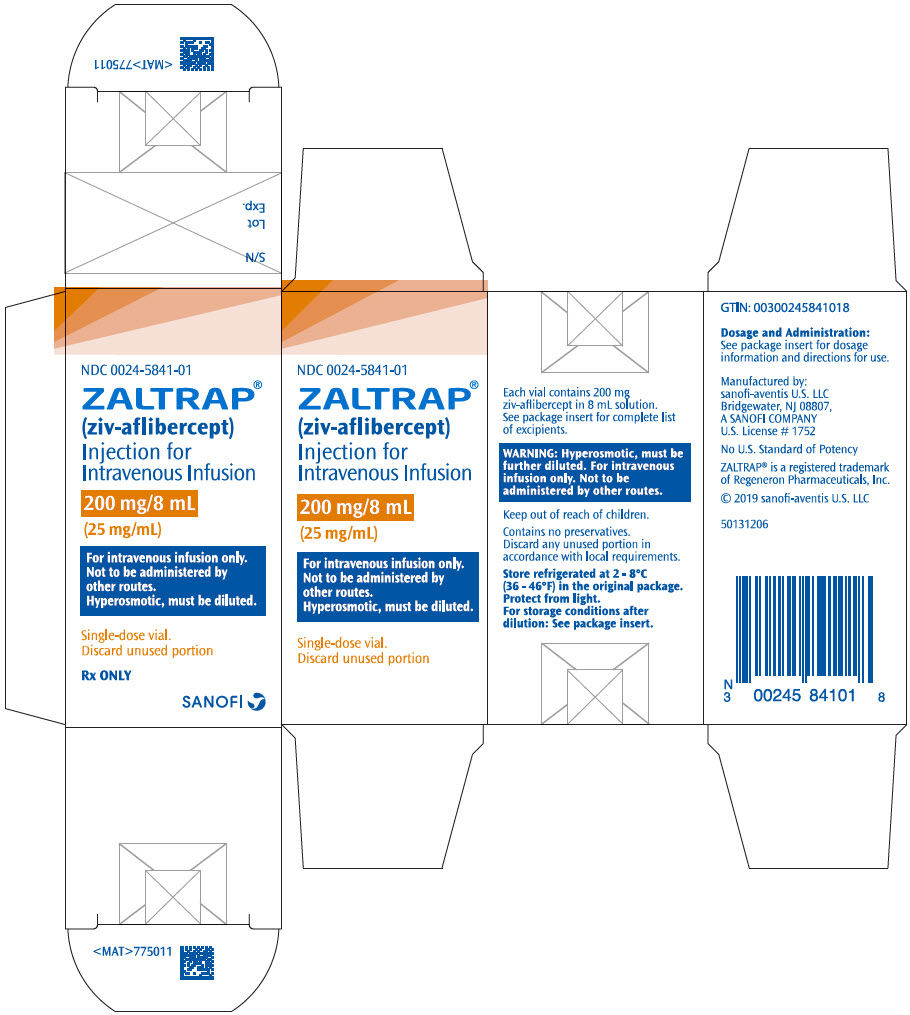
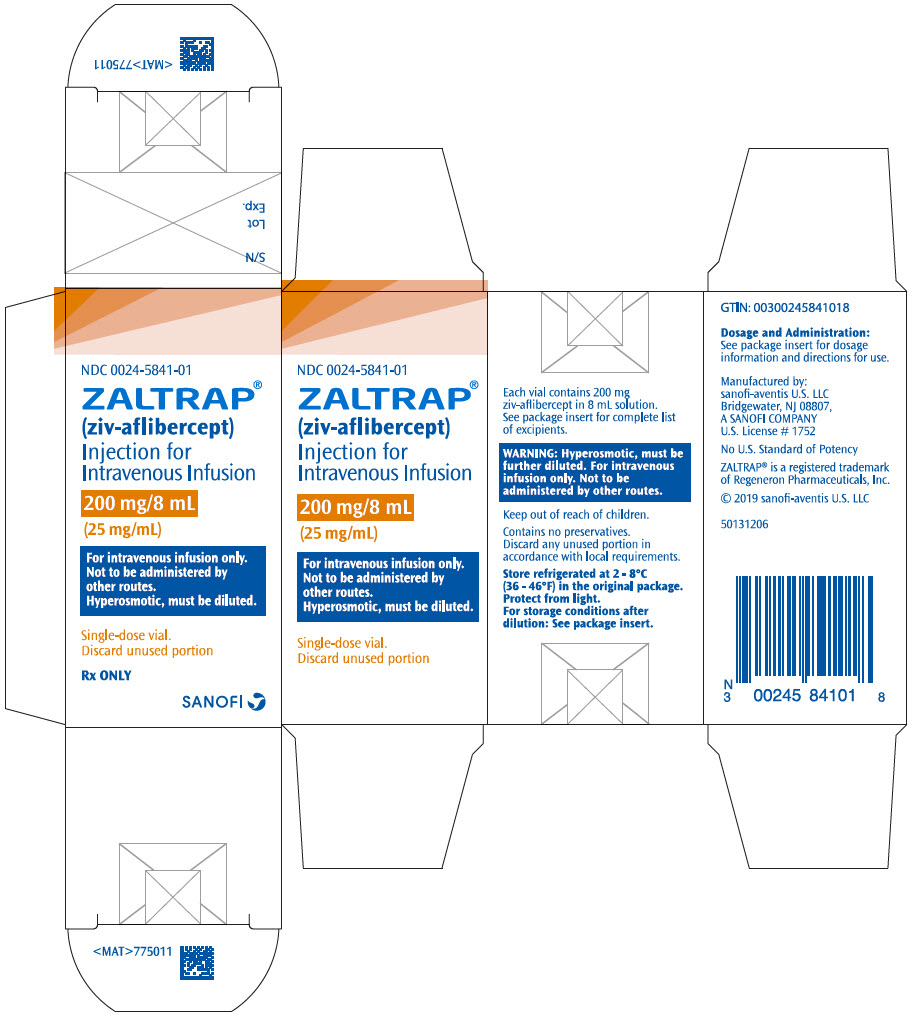
PRINCIPAL DISPLAY PANEL - 200 mg/8 mL Vial CartonNDC 0024-5841-01 - ZALTRAP® (ziv-aflibercept) Injection for - Intravenous Infusion - 200 mg/8 mL - (25 mg/mL) For intravenous infusion only. Not to be administered by - other routes. Hyperosmotic, must be ...
-
INGREDIENTS AND APPEARANCEProduct Information