Label: YONDELIS- trabectedin injection, powder, lyophilized, for solution
- NDC Code(s): 59676-610-01
- Packager: Janssen Products, LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use YONDELIS - ®safely and effectively. See full prescribing information for YONDELIS. YONDELIS (trabectedin) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEYONDELIS - ®is indicated for the treatment of patients with unresectable or metastatic liposarcoma or leiomyosarcoma who received a prior anthracycline-containing regimen - [see - Clinical ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dose is 1.5 mg/m - 2administered as an intravenous infusion over 24 hours through a central venous line every 21 days (3 weeks), until disease ...
-
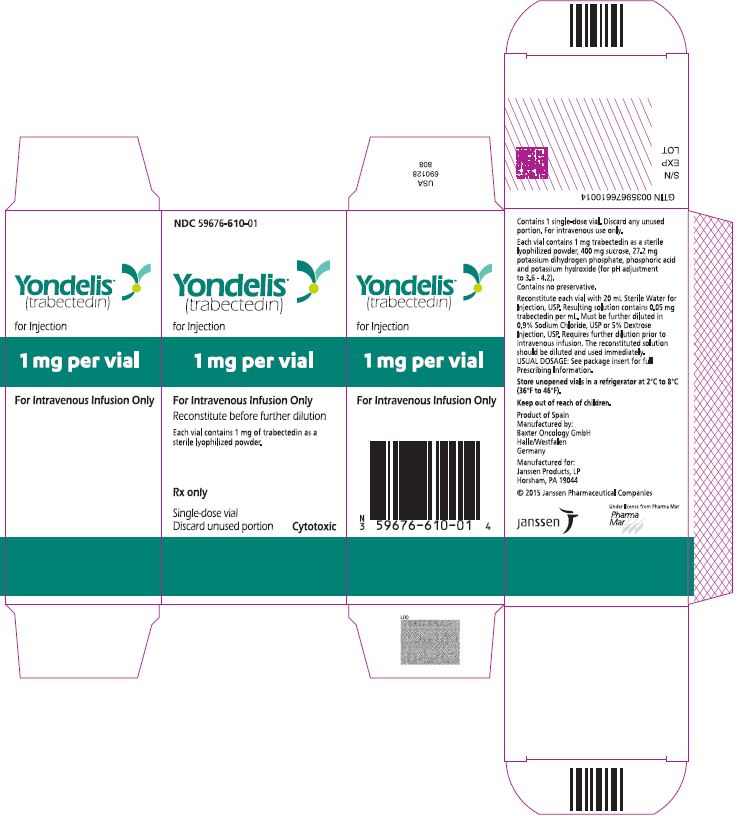
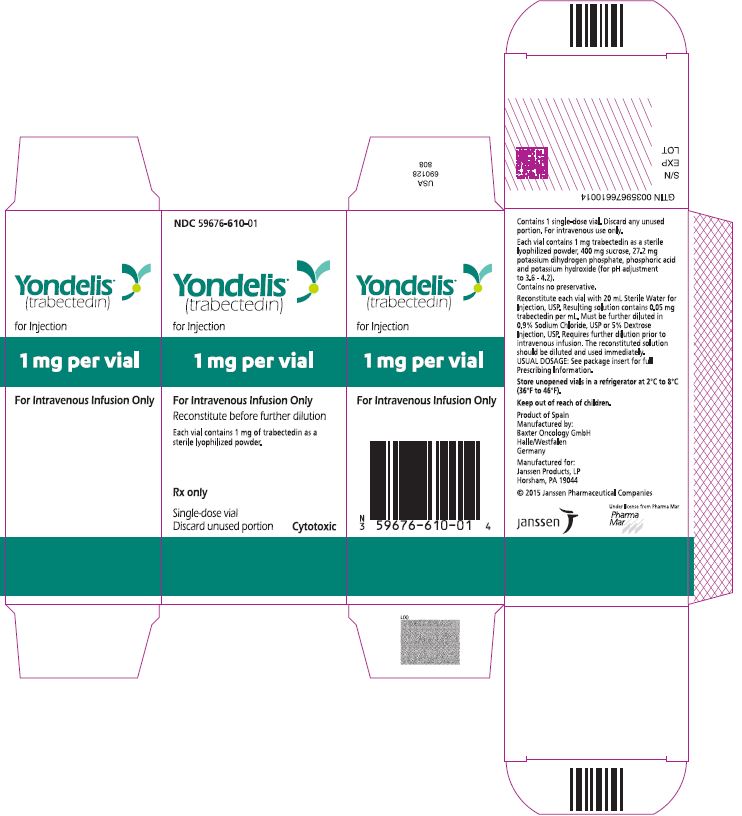
3 DOSAGE FORMS AND STRENGTHSFor injection: 1 mg, lyophilized powder in single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSYONDELIS is contraindicated in patients with known severe hypersensitivity, including anaphylaxis, to trabectedin.
-
5 WARNINGS AND PRECAUTIONS5.1 Neutropenic Sepsis - Neutropenic sepsis, including fatal cases, can occur with YONDELIS. In Trial ET743-SAR-3007, the incidence of Grade 3 or 4 neutropenia, based on laboratory values, in ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Anaphylaxis - [see - Contraindications (4)] Neutropenic Sepsis - [see ...
-
7 DRUG INTERACTIONS7.1 Effect of Cytochrome CYP3A Inhibitors - Coadministration of YONDELIS with ketoconazole, a strong CYP3A inhibitor, increased systemic exposure of trabectedin by 66%. Avoid using strong CYP3A ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, trabectedin can cause fetal harm when administered during pregnancy - [see - Clinical Pharmacology (12.1)] . There ...
-
10 OVERDOSAGEThere is no specific antidote for YONDELIS. Hemodialysis is not expected to enhance the elimination of YONDELIS because trabectedin is highly bound to plasma proteins (97%) and not significantly ...
-
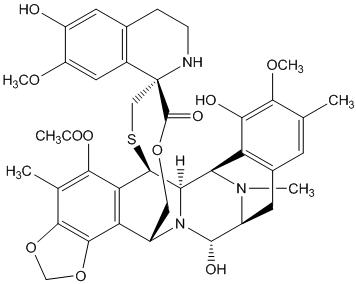
11 DESCRIPTIONTrabectedin is an alkylating drug with the chemical name (1' R,6 - R,6a - R,7 - R,13 - S,14 - S,16 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Trabectedin is an alkylating drug that binds guanine residues in the minor groove of DNA, forming adducts and resulting in a bending of the DNA helix towards the major ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Trabectedin is genotoxic in both - in vitroand - in vivostudies. Long-term carcinogenicity studies have not been performed ...
-
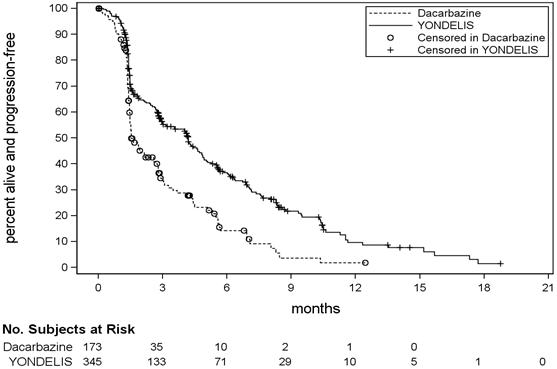
14 CLINICAL STUDIESThe clinical efficacy and safety of YONDELIS in patients with metastatic or recurrent leiomyosarcoma or liposarcoma were demonstrated in Trial ET743-SAR-3007 (NCT01343277), a randomized (2:1) ...
-
15 REFERENCES"OSHA Hazardous Drugs." OSHA.http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGYONDELIS is supplied in a single-dose glass vial containing 1 mg trabectedin. Each carton contains one vial (NDC: 59676-610-01). Storage and Handling - Store YONDELIS vials in a refrigerator at ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Myelosuppression: Inform patients of the risks of myelosuppression. Instruct patients to immediately contact ...
-
SPL UNCLASSIFIED SECTIONProduct of Spain - Manufactured by: Baxter Oncology GmbH - Halle/Westfalen Germany - Manufactured for: Janssen Products, LP - Horsham, PA - © 2015 Janssen Pharmaceutical ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - YONDELIS - ®(yon-DEL-ess) (trabectedin) for injection - This Patient Information has been approved by the U.S. Food and Drug ...
-
PRINCIPAL DISPLAY PANEL - 1 Vial CartonNDC 59676-610-01 - Yondelis - ® (trabectedin) for Injection - 1 mg per vial - For Intravenous Infusion Only - Reconstitute before further dilution - Each vial contains 1 mg of trabectedin ...
-
INGREDIENTS AND APPEARANCEProduct Information