Label: XOFIGO- radium ra 223 dichloride injection
- NDC Code(s): 50419-208-01
- Packager: Bayer HealthCare Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 10, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XOFIGO safely and effectively. See full prescribing information for XOFIGO. XOFIGO (radium Ra 223 dichloride) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEXofigo is indicated for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases and no known visceral metastatic disease.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The dose regimen of Xofigo is 55 kBq (1.49 microcurie) per kg body weight, given at 4 week intervals for 6 injections. Safety and efficacy beyond 6 injections with Xofigo ...
-
3 DOSAGE FORMS AND STRENGTHSXofigo (radium Ra 223 dichloride injection) is available in single-dose vials containing 6 mL of clear, colorless solution at a concentration of 1,100 kBq/mL (30 microcurie/mL) at the reference ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Bone Marrow Suppression - In the randomized trial, 2% of patients on the Xofigo arm experienced bone marrow failure or ongoing pancytopenia compared to no patients treated with placebo ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in another section of the label: • Bone Marrow Suppression [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONSNo formal clinical drug interaction studies have been performed. Subgroup analyses indicated that the concurrent use of bisphosphonates or calcium channel blockers did not affect the safety and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The safety and efficacy of Xofigo have not been established in females. Based on mechanism of action, Xofigo can cause fetal harm when administered to a pregnant ...
-
10 OVERDOSAGEThere have been no reports of inadvertent overdosing of Xofigo during clinical studies. There is no specific antidote. In the event of an inadvertent overdose of Xofigo, utilize general supportive ...
-
11 DESCRIPTIONRadium Ra 223 dichloride, an alpha particle-emitting pharmaceutical, is a radiotherapeutic drug. Xofigo is supplied as a clear, colorless, isotonic, and sterile solution to be administered ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The active moiety of Xofigo is the alpha particle-emitting isotope radium-223 (as radium Ra 223 dichloride), which mimics calcium and forms complexes with the bone ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to evaluate the carcinogenic potential of radium-223 dichloride. However, in repeat-dose ...
-
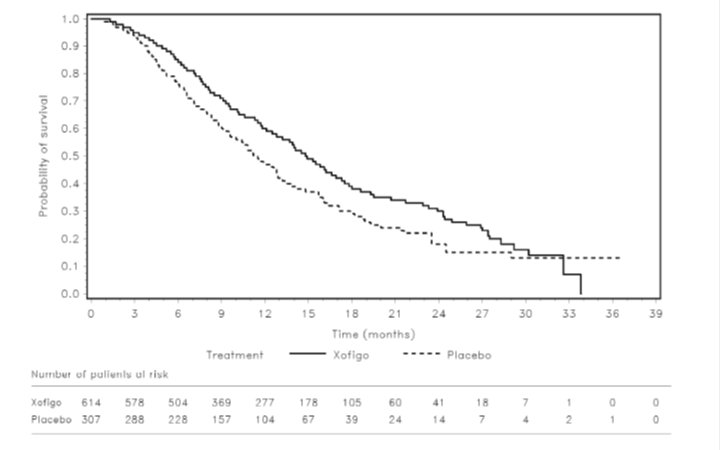
14 CLINICAL STUDIESThe efficacy and safety of Xofigo were evaluated in a double-blind, randomized, placebo-controlled phase 3 clinical trial of patients with castration-resistant prostate cancer with symptomatic ...
-
15 REFERENCES1. Radiation Emergency Medical Management. [REMM/National Library of Medicine Website.] http://www.remm.nlm.gov/int_contamination.htm#blockingagents - 2. International Commission on Radiological ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGXofigo (radium Ra 223 dichloride injection) is supplied in single-dose vials containing 6 mL of clear, colorless solution at a concentration of 1,100 kBq/mL (30 microcurie/mL) with a total ...
-
17 PATIENT COUNSELING INFORMATIONBone Marrow Suppression - Increased Fractures and Mortality in Combination with Abiraterone plus Prednisone/Prednisolone - Fluid Status - Instructions for Use/Handling - Embryo-Fetal ...
-
PRINCIPAL DISPLAY PANELNDC 50419-208-01 - 6 mL - Xofigo® radium Ra 223 dichloride injection - 1100 kBq/mL (30 microcurie/mL) For Intravenous Administration - Sterile - Single-Dose Vial: Discard Unused Portion - Manufactured ...
-
INGREDIENTS AND APPEARANCEProduct Information