Label: XENAZINE- tetrabenazine tablet
- NDC Code(s): 67386-421-01, 67386-422-01
- Packager: Lundbeck Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XENAZINE safely and effectively. See full prescribing information for XENAZINE. XENAZINE® (tetrabenazine) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DEPRESSION AND SUICIDALITY
XENAZINE can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington's disease. Anyone considering the use of XENAZINE must balance the risks of depression and suicidality with the clinical need for control of chorea. Close observation of patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior should accompany therapy. Patients, their caregivers, and families should be informed of the risk of depression and suicidality and should be instructed to report behaviors of concern promptly to the treating physician.
Particular caution should be exercised in treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in Huntington's disease. XENAZINE is contraindicated in patients who are actively suicidal, and in patients with untreated or inadequately treated depression [see Contraindications (4), Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEXENAZINE is indicated for the treatment of chorea associated with Huntington’s disease.
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Considerations - The chronic daily dose of XENAZINE used to treat chorea associated with Huntington’s disease (HD) is determined individually for each patient. When first ...
-
3 DOSAGE FORMS AND STRENGTHSXENAZINE tablets are available in the following strengths and packages: The 12.5 mg XENAZINE tablets are white, cylindrical, biplanar tablets with beveled edges, non-scored, embossed on one side ...
-
4 CONTRAINDICATIONSXENAZINE is contraindicated in patients: Who are actively suicidal, or in patients with untreated or inadequately treated depression [see Warnings and Precautions (5.1)]. With hepatic impairment ...
-
5 WARNINGS AND PRECAUTIONS5.1 Depression and Suicidality - Patients with Huntington’s disease are at increased risk for depression, suicidal ideation or behaviors (suicidality). XENAZINE increases the risk for ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Depression and Suicidality [see Warnings and Precautions (5.1)] Neuroleptic Malignant Syndrome (NMS ...
-
7 DRUG INTERACTIONS7.1 Strong CYP2D6 Inhibitors - In vitro studies indicate that α-HTBZ and β-HTBZ are substrates for CYP2D6. Strong CYP2D6 inhibitors (e.g., paroxetine, fluoxetine, quinidine) markedly increase ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of XENAZINE in pregnant women. Administration of tetrabenazine to rats ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - XENAZINE is not a controlled substance. 9.2 Abuse - Clinical trials did not reveal patients developed drug seeking behaviors, though these observations were not ...
-
10 OVERDOSAGEThree episodes of overdose occurred in the open-label trials performed in support of registration. Eight cases of overdose with XENAZINE have been reported in the literature. The dose of XENAZINE ...
-
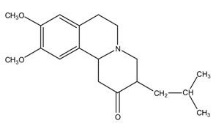
11 DESCRIPTIONXENAZINE (tetrabenazine) is a monoamine depletor for oral administration. The molecular weight of tetrabenazine is 317.43; the pKa is 6.51. Tetrabenazine is a hexahydro-dimethoxy-benzoquinolizine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which XENAZINE (tetrabenazine) exerts its anti-chorea effects is unknown but is believed to be related to its effect as a reversible depletor ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No increase in tumors was observed in p53+/- transgenic mice treated orally with tetrabenazine (5, 15, and 30 ...
-
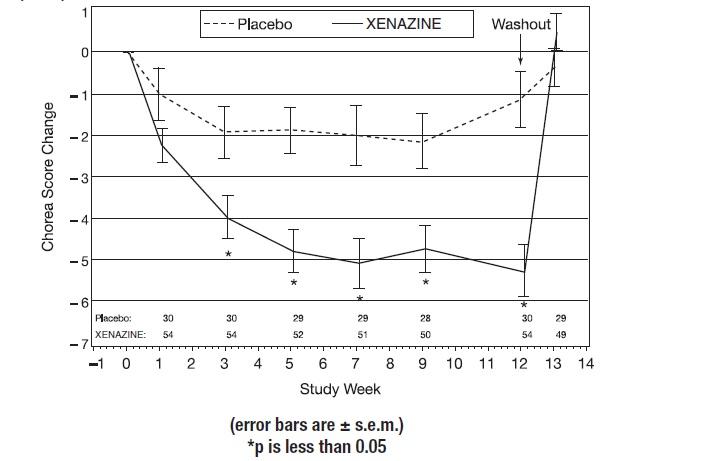
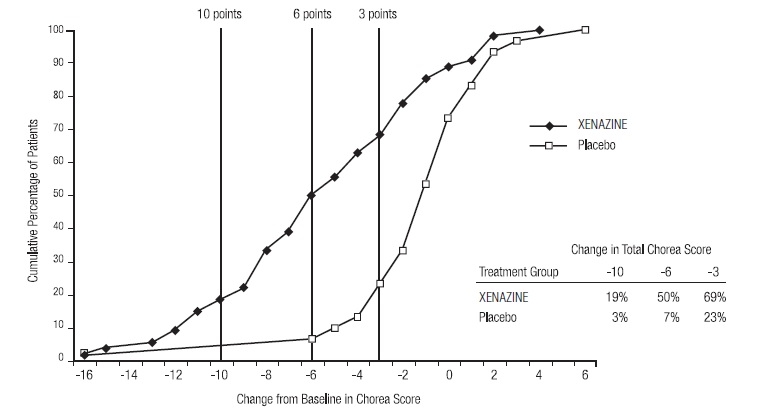
14 CLINICAL STUDIESStudy 1 - The efficacy of XENAZINE as a treatment for the chorea of Huntington’s disease was established primarily in a randomized, double-blind, placebo-controlled multi-center trial (Study ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - XENAZINE® (tetrabenazine) tablets are available in the following strengths and packages: The 12.5 mg XENAZINE tablets are white, cylindrical, biplanar tablets with beveled ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Risk of Suicidality - Inform patients and their families that XENAZINE may increase the risk of suicidal thinking ...
-
MEDICATION GUIDE
XENAZINE® (ZEN-uh-zeen) (tetrabenazine) Tablets - Read the Medication Guide that comes with XENAZINE before you start taking it and each time you refill the prescription. There may be new ...
-
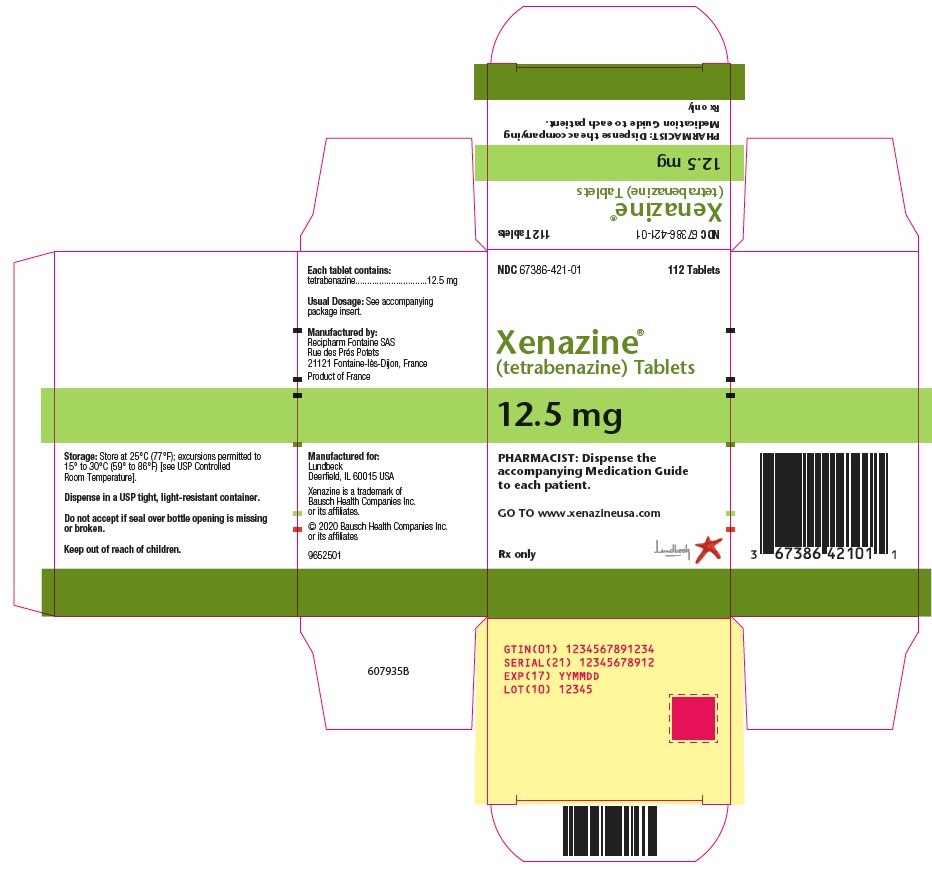
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 12.5 mg Carton - NDC 67386-421-01 - 112 Tablets - Xenazine® (tetrabenazine) Tablets - 12.5 mg - PHARMACIST: Dispense the - accompanying Medication Guide - to each ...
-
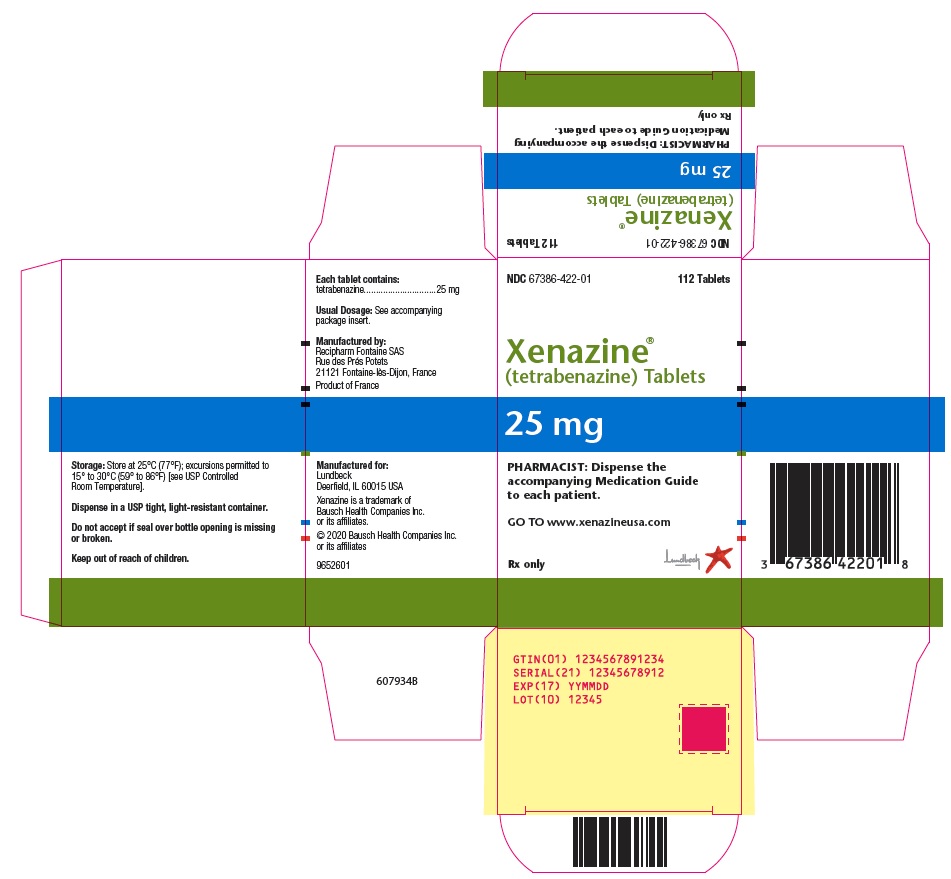
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 25 mg Carton - NDC 67386-422-01 - 112 Tablets - Xenazine® (tetrabenazine) Tablets - 25 mg - PHARMACIST: Dispense the - accompanying Medication Guide - to each ...
-
INGREDIENTS AND APPEARANCEProduct Information