The following adverse reactions are discussed in greater detail in other sections of the labeling:
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
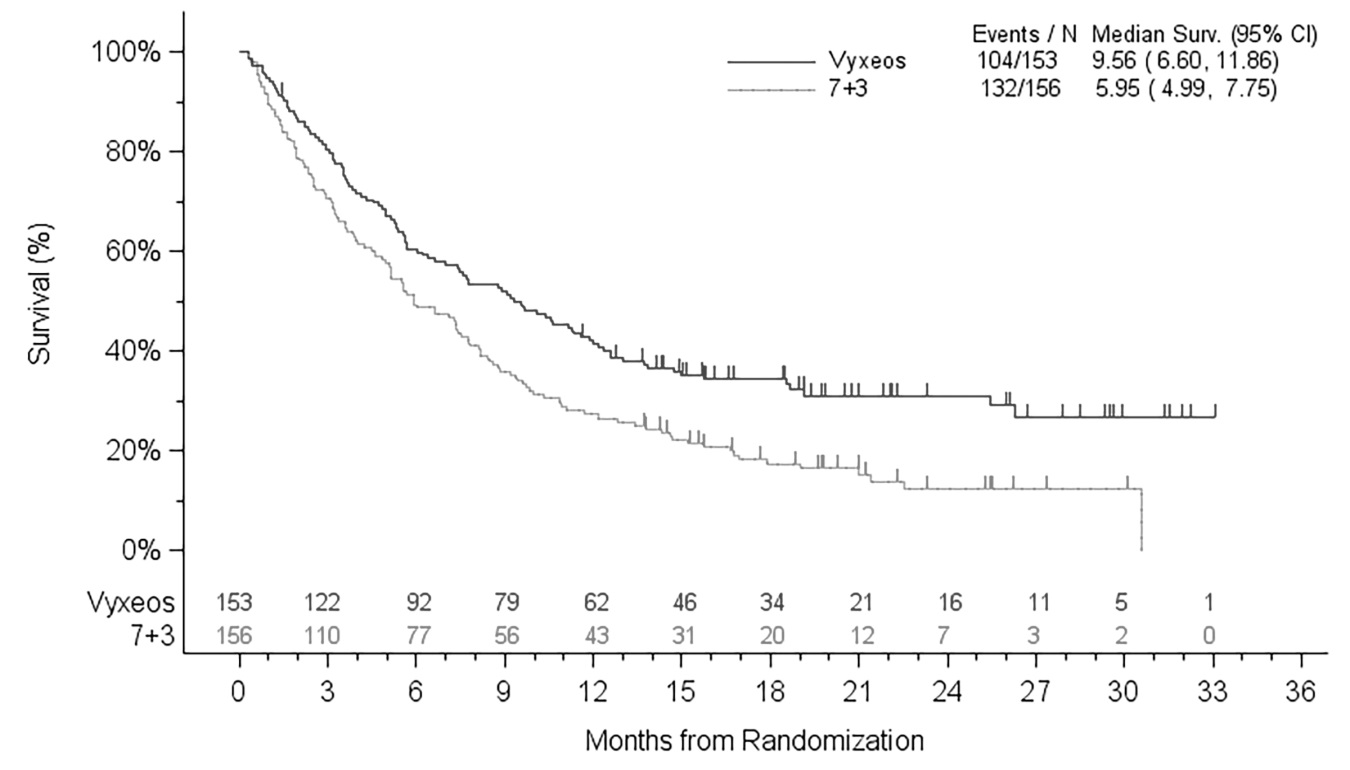
The safety of VYXEOS was determined in a randomized trial for adults with newly-diagnosed t‑AML or AML-MRC [see Clinical Studies (14)] which included 153 patients treated with VYXEOS and 151 patients treated with a standard combination of cytarabine and daunorubicin (7+3). At study entry, patients were required to have a LVEF of at least 50% and a prior lifetime cumulative anthracycline exposure less than 368 mg/m2 daunorubicin (or equivalent). On study, the median number of cycles administered was 2 (range, 1–4 cycles) on the VYXEOS arm and 1 (range, 1–4 cycles) on the control arm. The median cumulative daunorubicin dose was 189 mg/m2 (range, 44–337 mg/m2) on the VYXEOS arm and 186 mg/m2 (range, 44–532 mg/m2) on the control arm.
Nine patients each on the VYXEOS arm (6%) and the control arm (6%) had a fatal adverse reaction on treatment or within 30 days of therapy that was not in the setting of progressive disease. Fatal adverse reactions on the VYXEOS arm included infection, CNS hemorrhage, and respiratory failure. Overall, all-cause day-30 mortality was 6% in the VYXEOS arm and 11% in the control arm. During the first 60 days of the study, 14% (21/153) of patients died in the VYXEOS arm vs. 21% (32/151) of patients in the 7+3 treatment group.
The most common serious adverse reactions (incidence ≥ 5%) on the VYXEOS arm were dyspnea, myocardial toxicity, sepsis, pneumonia, febrile neutropenia, bacteremia and hemorrhage. Adverse reactions led to discontinuation of VYXEOS in 18% (28/153) of patients, and 13% (20/151) in the control arm. The adverse reactions leading to discontinuation on the VYXEOS arm included prolonged cytopenias, infection, cardiotoxicity, respiratory failure, hemorrhage (GI and CNS), renal insufficiency, colitis, and generalized medical deterioration. The most common adverse reactions (incidence ≥ 25%) in patients on the VYXEOS arm were hemorrhagic events, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting. The incidences of common adverse drug reactions during the induction phase in Study 1 are presented in Table 3.
a Grouped terms: Hemorrhage: Anal hemorrhage, Blood blister, Blood urine present, Breast hematoma, Catheter site bruise, Catheter site hemorrhage, Central nervous system hemorrhage, Cerebral hematoma, Cerebral hemorrhage, Coagulopathy, Conjunctival hemorrhage, Contusion, Ecchymosis, Enterocolitis hemorrhagic, Epistaxis, Gastric hemorrhage, Gastrointestinal hemorrhage, Gingival bleeding, Hematemesis, Hematochezia, Hematoma, Hematuria, Hemoptysis, Hemorrhage, Hemorrhage intracranial, Hemorrhage subcutaneous, Hemorrhage urinary tract, Hemorrhoidal hemorrhage, Lip hematoma, Lip hemorrhage, Lower gastrointestinal hemorrhage, Melena, Mouth hemorrhage, Mucosal hemorrhage, Periorbital hematoma, Periorbital hemorrhage, Pharyngeal hematoma, Pharyngeal hemorrhage, Post procedural contusion, Post procedural hematoma, Post procedural hemorrhage, Pulmonary alveolar hemorrhage, Pulmonary hemorrhage, Purpura, Rectal hemorrhage, Retinal hemorrhage, Scleral hemorrhage, Scrotal hematoma, Skin ulcer hemorrhage, Small intestinal hemorrhage, Stomatitis hemorrhagic, Subdural hematoma, Subdural hemorrhage, Subgaleal hematoma, Tongue hemorrhage, Traumatic hematoma, Upper gastrointestinal hemorrhage, Urethral hemorrhage, Vaginal hemorrhage, Vessel puncture site hemorrhage, Vitreous hemorrhage; Rash: Dermatitis, Dermatitis acneiform, Dermatitis allergic, Dermatitis contact, Eczema, Erythema nodosum, Exfoliative rash, Psoriasis, Rash, Rash erythematous, Rash follicular, Rash generalized, Rash macular, Rash maculo-papular, Rash papular, Rash pruritic, Rash pustular, Skin exfoliation; Edema: Face edema, Fluid overload, Fluid retention, Generalized edema, Localized edema, Edema, Edema peripheral, Penile edema, Scrotal edema, Swelling, Swelling face; Diarrhea/Colitis: Cecitis, Colitis, Diarrhea, Enterocolitis, Ileitis, Neutropenic colitis, Enteritis, Enterocolitis; Mucositis: Anal erosion, Anorectal discomfort, Duodenitis, Gastric ulcer, Gastrointestinal inflammation, Gingival pain, Gingival swelling, Gingivitis, Glossodynia, Laryngeal inflammation, Lip ulceration, Mouth ulceration, Mucosal inflammation, Mucosal ulceration, Odynophagia, Edema mouth, Esophageal ulcer, Esophagitis, Oral mucosa erosion, Oral mucosal blistering, Oral mucosal erythema, Pharyngeal ulceration, Proctalgia, Proctitis, Rectal ulcer, Stomatitis, Tongue ulceration, Oropharyngeal pain, Oral pain, Oropharyngeal discomfort, Pharyngeal erythema; Musculoskeletal pain: Arthralgia, Back pain, Bone pain, Coccydynia, Limb discomfort, Musculoskeletal chest pain, Musculoskeletal pain, Myalgia, Neck pain, Pain in extremity, Pain in jaw; Abdominal pain: Abdominal pain, Abdominal distension, Abdominal pain upper, Abdominal discomfort, Abdominal pain lower, Abdominal tenderness; Cough: Cough, Productive Cough; Headache: Headache, Sinus Headache; Dyspnea: Acute respiratory distress syndrome, Acute respiratory failure, Bronchospasm, Dyspnea, Dyspnea exertional, Respiratory distress, Respiratory failure, Wheezing; Fatigue: Fatigue, Asthenia; Arrhythmia: Arrhythmia, Arrhythmia supraventricular, Atrial fibrillation, Atrial flutter, Atrial tachycardia, Atrioventricular block first degree, Atrioventricular block second degree, Bradycardia, Bundle branch block right, Extrasystoles, Heart rate increased, Nodal arrhythmia, Nodal rhythm, Sinus arrest, Sinus arrhythmia, Sinus bradycardia, Sinus tachycardia, Supraventricular tachycardia, Tachycardia, Ventricular extrasystoles, Ventricular tachycardia; Pneumonia (excluding fungal): Lung consolidation, Lung infection, Lung infiltration, Pneumonia, Pneumonia aspiration, Pneumonia bacterial, Pneumonia klebsiella, Pneumonia pseudomonas aeruginosa, Pneumonia viral; Sleep disorders: Abnormal dreams, Insomnia, Nightmare, Sleep apnea syndrome, Sleep disorder; Bacteremia (excluding sepsis): Bacillus test positive, Bacteremia, Bacteroides bacteremia, Corynebacterium test positive, Enterobacter bacteremia, Enterococcal bacteremia, Enterococcus test positive, Escherichia bacteremia, Klebsiella bacteremia, Pseudomonal bacteremia, Pseudomonas test positive, Staphylococcal bacteremia, Staphylococcus test positive, Stomatococcus test positive, Streptococcal bacteremia, Streptococcus test positive, Escherichia test positive, Klebsiella test positive; Vomiting: Retching, Vomiting; Hypotension: Hypotension, Orthostatic hypotension; Non-conduction cardiotoxicity: Acute coronary syndrome, Acute endocarditis, Acute myocardial infarction, Angina pectoris, Aortic valve incompetence, Cardiac arrest, Cardiac failure, Cardiac failure congestive, Cardiac murmur, Cardiogenic shock, Cardiomegaly, Cardiomyopathy, Cardiotoxicity, Cytotoxic cardiomyopathy, Diastolic dysfunction, Dilatation atrial, Dilatation ventricular, Ejection fraction decreased, Endocarditis, Left ventricular dysfunction, Mitral valve incompetence, Myocardial infarction, Pericardial effusion, Pericarditis, Restrictive cardiomyopathy, Right ventricular hypertrophy; Dizziness: Dizziness, Dizziness postural, Dizziness exertional; Fungal infection: Aspergillosis, Bronchopulmonary aspergillosis, Candida test positive, Candidiasis, Fungemia, Fungal infection, Fungal skin infection, Intertrigo candida, Lower respiratory tract infection fungal, Oral candidiasis, Pneumonia fungal, Pulmonary mycosis, Sinusitis fungal, Skin candida, Tinea cruris, Tinea infection, Vulvovaginal mycotic infection, Wound infection fungal, Zygomycosis, Mycotic aneurysm; Hypertension: Blood pressure increased, Hypertension; Hypoxia: Hypoxia, Oxygen saturation decreased; Upper respiratory infection (excluding fungal): Acute sinusitis, Chronic sinusitis, Increased upper airway secretion, Nasal congestion, Pharyngitis, Rhinitis, Rhinorrhea, Sinus congestion, Sinusitis, Sinusitis bacterial, Upper respiratory tract congestion, Upper respiratory tract infection, Upper-airway cough syndrome, Viral upper respiratory tract infection; Chest pain: Chest discomfort, Chest pain, Non-cardiac chest pain, Pleuritic pain; Catheter/device/injection site reaction: Catheter site discharge, Catheter site erosion, Catheter site erythema, Catheter site inflammation, Catheter site edema, Catheter site pain, Catheter site pruritus, Catheter site rash, Infusion site edema, Infusion site pain, Infusion site vesicles, Catheter site related reaction; Delirium: Cognitive disorder, Confusional state, Delirium; Pruritis: Anal pruritis, Ear pruritis, Pruritis, Pruritis generalized; Sepsis (excluding fungal): Enterobacter sepsis, Escherichia sepsis, Klebsiella sepsis, Neutropenic sepsis, Sepsis, Septic shock, Staphylococcal sepsis, Streptococcal sepsis, Urosepsis, Viral sepsis; Renal insufficiency: Acute prerenal failure, Azotemia, Oliguria, Renal failure, Renal failure acute, Renal failure chronic; Transfusion reactions: Allergic transfusion reaction, Febrile non-hemolytic transfusion reaction, Transfusion reaction; Visual impairment (except bleeding): Photophobia, Photopsia, Photosensitivity reaction, Retinal tear, Scintillating scotoma, Uveitis, Vision blurred, Visual acuity reduced, Visual impairment, Vitreous detachment, Vitreous floaters
b Adverse reactions were graded using NCI CTCAE version 3.0.
During the consolidation phase (both consolidation cycles pooled) the two most common adverse reactions on the VYXEOS arm are the same as those during induction, hemorrhagic events and febrile neutropenia. These occurred at lower rates in the pooled consolidation phase (43% and 29%, respectively), compared to the induction phase. All of the common adverse reactions (≥ 10% incidence in the VYXEOS arm) seen in the pooled consolidation phase were also seen in the induction phase. These occurred at lower incidence in the consolidation phase, with the exception of chills, dizziness and pyrexia, where the incidences were relatively similar across the induction and consolidation cycles.
Other notable adverse drug reactions that occurred in less than 10% of patients treated with VYXEOS during induction or consolidation included:
- •
- Ear and labyrinth disorders: Deafness, Deafness unilateral
- •
- Eye Disorders: Eye conjunctivitis, Dry eye, Eye edema, Eye swelling, Eye irritation, Eye pain, Ocular discomfort, Ocular hyperemia, Periorbital edema, Scleral hyperemia
- •
- Gastrointestinal disorders: Dyspepsia
- •
- Psychiatric disorders: Hallucinations
- •
- Respiratory, thoracic and mediastinal disorders: Pneumonitis
Laboratory Abnormalities
All patients developed severe neutropenia, thrombocytopenia, and anemia. See Table 4 for the incidences of Grade 3 thrombocytopenia and Grade 4 neutropenia that were prolonged in the absence of active leukemia.
a Platelets < 50 Gi/L or neutrophils < 0.5 Gi/L lasting past cycle day 42 in the absence of active leukemia.
b Patients receiving at least 1 consolidation.
Grade 3-4 chemistry abnormalities occurring in greater than 5% of VYXEOS treated patients in Study 1 are presented in Table 5.
a Graded using NCI CTCAE version 3.0.