Label: VYNDAQEL- tafamidis meglumine capsule, liquid filled
VYNDAMAX- tafamidis capsule, liquid filled
- NDC Code(s): 0069-1975-12, 0069-1975-40, 0069-8730-01, 0069-8730-30
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 1, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VYNDAQEL and VYNDAMAX safely and effectively. See full prescribing information for VYNDAQEL and VYNDAMAX. VYNDAQEL® (tafamidis ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGEVYNDAQEL and VYNDAMAX are indicated for the treatment of the cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality and ...
-
2. DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage is either VYNDAQEL 80 mg (four 20-mg tafamidis meglumine capsules) orally once daily or VYNDAMAX 61 mg (one 61-mg tafamidis capsule) orally once ...
-
3. DOSAGE FORMS AND STRENGTHSVYNDAQEL is available as: • tafamidis meglumine 20 mg: yellow, opaque, oblong capsule, printed with "VYN 20" in red. VYNDAMAX is available as: • tafamidis 61 mg: reddish brown, opaque, oblong ...
-
4. CONTRAINDICATIONSNone.
-
6. ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7. DRUG INTERACTIONS7.1 BCRP Substrates - Tafamidis inhibits breast cancer resistant protein (BCRP) in humans [see Clinical Pharmacology (12.3)]. Coadministration of tafamidis and drugs that are BCRP substrates may ...
-
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies, VYNDAQEL and VYNDAMAX may cause fetal harm when administered to a pregnant woman. However, limited available human data ...
-
10. OVERDOSAGEThere is minimal clinical experience with overdose. During clinical trials, two patients accidentally ingested a single VYNDAQEL dose of 160 mg without adverse events. The highest dose of ...
-
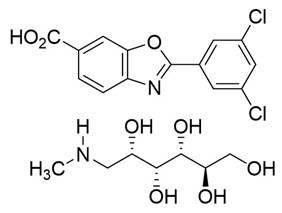
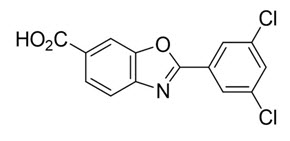
11. DESCRIPTIONVYNDAQEL (tafamidis meglumine) and VYNDAMAX (tafamidis) contain tafamidis as the active moiety, which is a selective stabilizer of transthyretin. The chemical name of tafamidis meglumine is ...
-
12. CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tafamidis is a selective stabilizer of TTR. Tafamidis binds to TTR at the thyroxine binding sites, stabilizing the tetramer and slowing dissociation into monomers, the ...
-
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - There was no evidence of an increased incidence of neoplasia in the transgenic (Tg)-rasH2 mouse following repeated ...
-
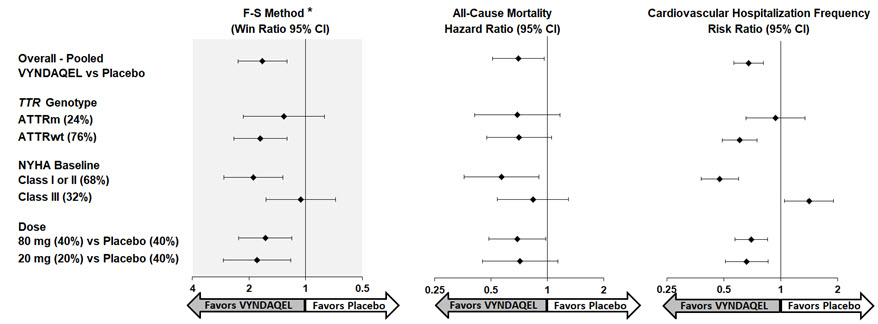
14. CLINICAL STUDIESEfficacy was demonstrated in a multicenter, international, randomized, double-blind, placebo-controlled study in 441 patients with wild-type or hereditary ATTR-CM (NCT01994889). Patients were ...
-
16. HOW SUPPLIED/STORAGE AND HANDLINGVYNDAQEL 20-mg (tafamidis meglumine) soft gelatin capsules are yellow, opaque, oblong, and printed with "VYN 20" in red and supplied in the following package configurations: VYNDAQEL ...
-
17. PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy - Report pregnancies to the Pfizer reporting line at 1-800-438-1985. Advise pregnant women and ...
-
SPL UNCLASSIFIED SECTIONLAB-0497-6.0
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration - Issued: 10/2023 - PATIENT INFORMATION - VYNDAQEL® (VIN-duh-kel) (tafamidis ...
-
PRINCIPAL DISPLAY PANEL - 20 mg Capsule Blister CardNDC 0069-1975-12 - Vyndaqel® (tafamidis meglumine) capsules - 20 mg per capsule - Pfizer Labs. LOT/EXP - PEEL & PUSH
-
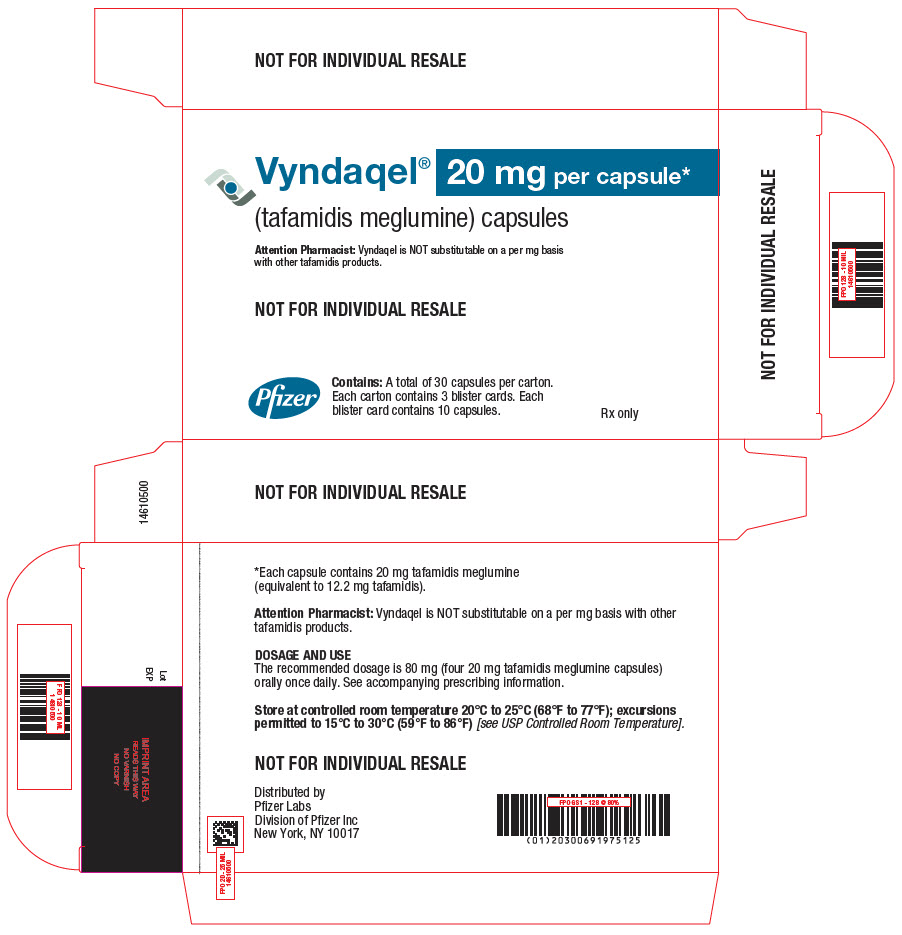
PRINCIPAL DISPLAY PANEL - 20 mg Capsule Blister Card CartonVyndaqel® (tafamidis meglumine) capsules - 20 mg per capsule* Attention Pharmacist: Vyndaqel is NOT substitutable on a per mg basis - with other tafamidis products. NOT FOR INDIVIDUAL ...
-
PRINCIPAL DISPLAY PANEL - 20 mg Capsule Blister Card Carton CartonNDC 0069-1975-40 - Vyndaqel® (tafamidis meglumine) capsules - 20 mg per capsule* Attention Pharmacist: Vyndaqel is NOT substitutable on a per mg basis with other - tafamidis products. Pfizer - Contains ...
-
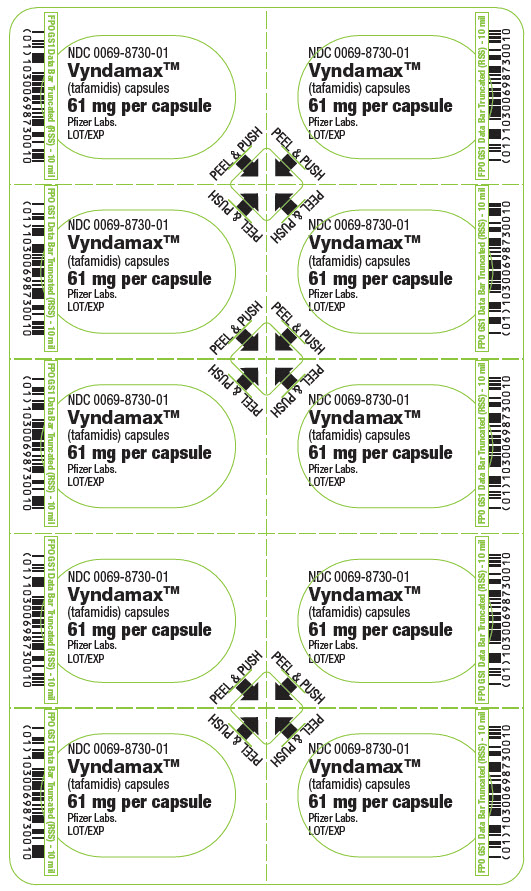
PRINCIPAL DISPLAY PANEL - 61 mg Capsule Blister CardNDC 0069-8730-01 - Vyndamax™ (tafamidis) capsules - 61 mg per capsule - Pfizer Labs. LOT/EXP - PEEL & PUSH
-
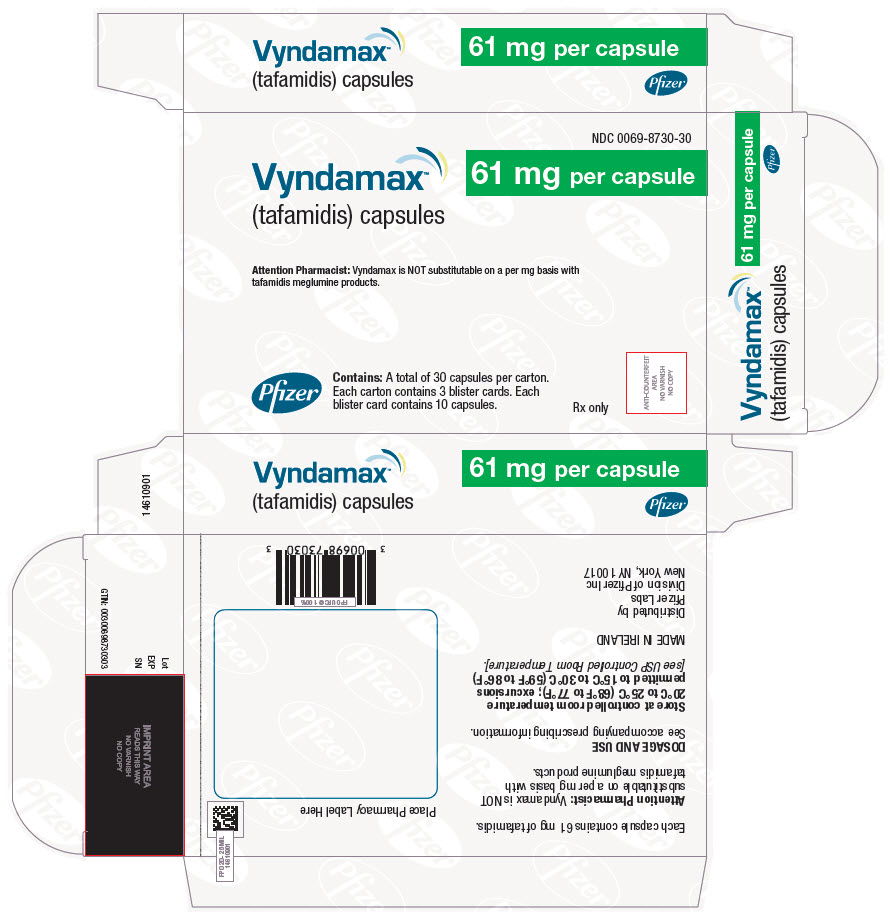
PRINCIPAL DISPLAY PANEL - 61 mg Capsule Blister Card CartonNDC 0069-8730-30 - Vyndamax™ (tafamidis) capsules - 61 mg per capsule - Attention Pharmacist: Vyndamax is NOT substitutable on a per mg basis with - tafamidis meglumine products. Pfizer - Contains: A total ...
-
INGREDIENTS AND APPEARANCEProduct Information