Label: VPRIV- velaglucerase alfa injection, powder, lyophilized, for solution
- NDC Code(s): 54092-701-04
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VPRIV safely and effectively. See full prescribing information for VPRIV. VPRIV® (velaglucerase alfa) for injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement and after extended duration of therapy.
Initiate VPRIV in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue VPRIV and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEVPRIV is indicated for long-term enzyme replacement therapy (ERT) for patients with type 1 Gaucher disease.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommendations Prior to VPRIV treatment - Administration of VPRIV should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions including ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 400 units of a sterile, white to off-white, lyophilized powder in single-dose vials for reconstitution and dilution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions Including Anaphylaxis - Life-threatening hypersensitivity reactions, including anaphylaxis, have occurred in patients treated with enzyme replacement therapies ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data on use of velaglucerase alfa in pregnant women includes more than 300 pregnancies reported from the pharmacovigilance database and published ...
-
11 DESCRIPTIONVelaglucerase alfa is a hydrolytic lysosomal glucocerebroside-specific enzyme produced by gene activation technology in a human fibroblast cell line. Velaglucerase alfa is a glycoprotein of 497 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Gaucher disease is an autosomal recessive disorder caused by mutations in the GBA gene, which results in a deficiency of the lysosomal enzyme beta-glucocerebrosidase ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with ...
-
14 CLINICAL STUDIES14.1 Overview of Clinical Studies of VPRIV for Gaucher Disease - The efficacy of VPRIV was assessed in three clinical trials in a total of 99 patients with type 1 Gaucher disease: 82 patients age ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - VPRIV (velaglucerase alfa) for injection is a sterile, preservative free, white to off-white lyophilized powder requiring reconstitution and further dilution prior to use. It is ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity Reactions Including Anaphylaxis - Advise patients of the following: Life-threatening hypersensitivity reactions, including anaphylaxis may occur with VPRIV treatment. Anaphylaxis ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Takeda Pharmaceuticals U.S.A., Inc. Cambridge, MA 02142 - US License Number: 1898 - VPRIV and are registered trademarks of Shire Human Genetic Therapies, Inc. ©2024 Takeda ...
-
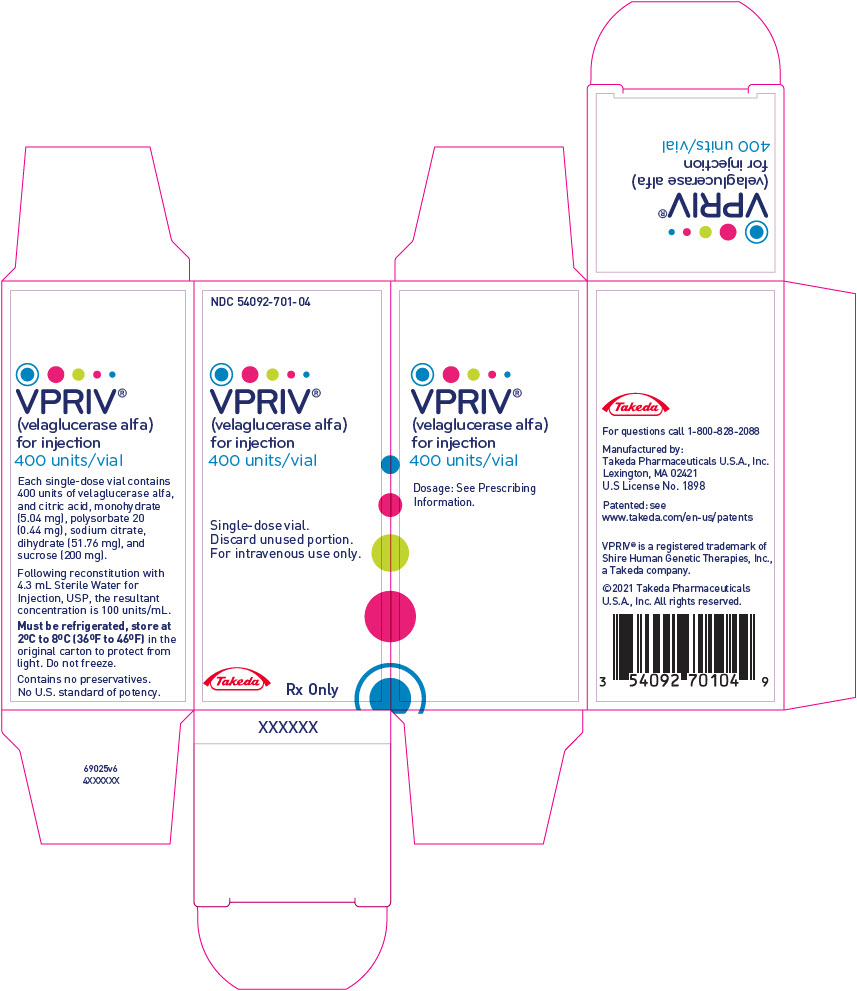
PRINCIPAL DISPLAY PANEL - 400 Unit Vial BoxNDC 54092-701-04 - VPRIV® (velaglucerase alfa) for injection - 400 units/vial - Single-dose vial. Discard unused portion. For intravenous use only. Takeda - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information

 are registered trademarks of Shire Human Genetic Therapies, Inc.
are registered trademarks of Shire Human Genetic Therapies, Inc.