Label: VOLUMEX- iodinated i-131 albumin injection, solution
- NDC Code(s): 50914-7720-8
- Packager: Iso-Tex Diagnostics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
DESCRIPTION
Volumex (Iodinated I 131 Albumin Injection) is a diagnostic radiopharmaceutical containing iodinated I 131 albumin for intravenous use. Each mL of sterile, nonpyrogenic, aqueous, colorless to very ...
-
PHYSICAL CHARACTERISTICS
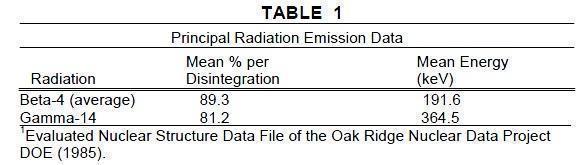
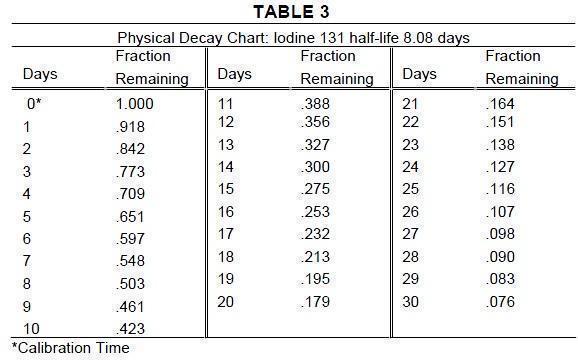
Iodine 131 decays by beta and gamma emissions with a physical half-life of 8.08 days.1 - Photons that are useful for detection and imaging studies are listed in Table 1. EXTERNAL ...
-
CLINICAL PHARMACOLOGY
Following intravenous injection, radioiodinated albumin human is uniformly distributed throughout the intravascular pool within 10 minutes; extravascular distribution takes place more slowly ...
-
INDICATIONS AND USAGE
Volumex (Iodinated I 131 Allbumin injection) is indicated for use in determinations of total blood and plasma volumes and in protein turnover studies
-
CONTRAINDICATIONS
None known.
-
WARNINGS
A few instances of hyperpyrexia and aseptic (chemical) meningeal irritation have been reported - with the use of iodinated I 131 albumin in cisternography. Iodinated I 131 Albumin Injection is not ...
-
PRECAUTIONS
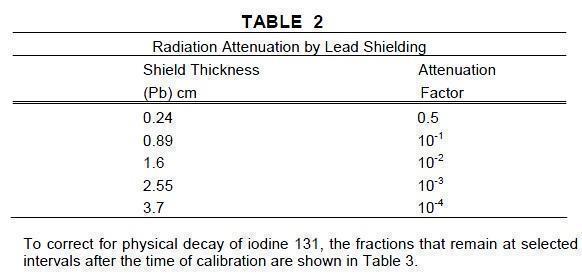
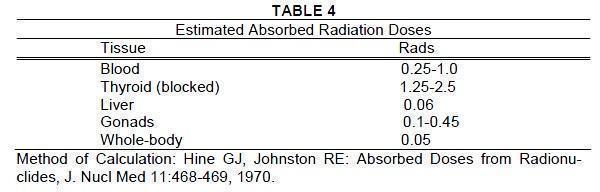
General - In the use of any radioactive material, care should be taken to insure minimum radiation exposure to the patient and occupational workers consistent with proper patient management ...
-
ADVERSE REACTIONS
Although the immunological properties of albumin human are believed to be virtually unaltered by the iodination process, there is a theoretical possibility that allergic reactions may occur in ...
-
DOSAGE AND ADMINISTRATION
Volumex (Iodinated I 131 Albumin Injection) is administered intravenously. Parenteral drug products should be inspected visually for particulate matter and abnormal coloration prior to ...
-
HOW SUPPLIED
Volumex (Iodinated I 131 Albumin Injection USP) is available in single unit dose syringes containing 25 microcuries of activity in one milliliter per syringe on the date of calibration. Each ...
-
Storage
Store in a refrigerator, 2-8°C (36-46°F)
-
SPL UNCLASSIFIED SECTIONThis radiopharmaceutical is licensed by the Texas Department of Health, Bureau of Radiation Control for distribution to persons licensed - pursuant to 41.26 (b) and Appendix 41-C, Group I and Group ...
-
SPL UNCLASSIFIED SECTIONManufactured for Daxor Corporation - 350 Fifth Avenue, Suite 7120 · New York, NY 10118 · 212-244-0804 - by Iso-Tex Diagnostics, Inc., Friendswood, Texas 77546
-
Packaging

-
INGREDIENTS AND APPEARANCEProduct Information