Label: VIVACAINE- bupivacaine hydrochloride and epinephrine bitartrate injection, solution
- NDC Code(s): 0362-9011-50
- Packager: Septodont, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - THIS SOLUTION IS INTENDED FOR DENTAL USE.
-
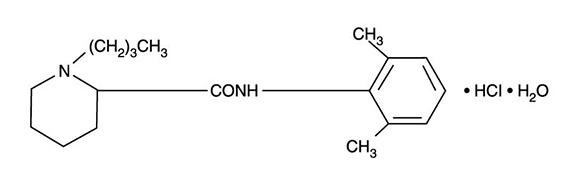
DESCRIPTIONBupivacaine hydrochloride is (±) -1-Butyl-2´, 6´-pipecoloxylidide monohydrochloride, monohydrate, a white crystalline powder that is freely soluble in 95 percent ethanol, soluble in water, and ...
-
CLINICAL PHARMACOLOGYBupivacaine stabilizes the neuronal membrane and prevents the initiation and transmission of nerve impulses, thereby effecting local anesthesia. The onset of action following dental injections is ...
-
INDICATIONS AND USAGEVivacaine® (bupivacaine hydrochloride and epinephrine injection, USP) is indicated for the production of local anesthesia for dental procedures by infiltration injection or nerve block in ...
-
CONTRAINDICATIONSVivacaine® (bupivacaine hydrochloride and epinephrine injection, USP) is contraindicated in patients with a known hypersensitivity to it or to any local anesthetic agent of the amide type or to ...
-
WARNINGSLOCAL ANESTHETICS SHOULD BE EMPLOYED ONLY BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE EMERGENCIES WHICH MIGHT ARISE FROM THE BLOCK TO BE ...
-
PRECAUTIONSThe safety and effectiveness of local anesthetics depend upon proper dosage, correct technique, adequate precautions, and readiness for emergencies. The lowest dosage that gives effective ...
-
ADVERSE REACTIONSReactions to Vivacaine® (bupivacaine hydrochloride and epinephrine injection, USP) are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse ...
-
DOSAGE AND ADMINISTRATIONAs with all local anesthetics, the dosage varies and depends upon the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, individual tolerance ...
-
HOW SUPPLIEDStore at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature]. Protect from light. Do not permit to freeze. Vivacaine® (bupivacaine hydrochloride and epinephrine injection, USP) ...
-
SPL UNCLASSIFIED SECTIONManufactured for - Septodont Inc., Lancaster, PA, USA 17601 - by NOVOCOL Pharmaceutical of Canada, Inc., Cambridge, Ontario, Canada N1R 6X3 ...
-
PRINCIPAL DISPLAY PANEL - 50 Cartridge CartonNDC 0362-9011-50 - Vivacaine® Bupivacaine HCl 0.5% (5 mg/mL) and Epinephrine 1:200,000 Injection (as bitartrate) (Bupivacaine Hydrochloride and Epinephrine Injection, USP) WARNINGS:CONTAINS ...
-
INGREDIENTS AND APPEARANCEProduct Information