Label: VIOKACE- pancrelipase tablet
- NDC Code(s): 73562-104-10, 73562-208-10
- Packager: Aimmune Therapeutics, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VIOKACE safely and effectively. See full prescribing information for VIOKACE. VIOKACE® (pancrelipase) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE VIOKACE, in combination with a proton pump inhibitor, is indicated for the treatment of exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatectomy in adults.
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Dosing Information - VIOKACE is a mixture of enzymes including lipases, proteases, and amylases. VIOKACE dosing is based on lipase units. • Administer VIOKACE with a proton pump ...
-
3 DOSAGE FORMS AND STRENGTHS Tablets are available in the following strengths: • 10,440 USP units of lipase; 39,150 USP units of protease; and 39,150 USP units of amylase as a tan, round, biconvex tablet with VIO9111 ...
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Fibrosing Colonopathy - Fibrosing colonopathy has been reported following treatment with pancreatic enzyme products. Fibrosing colonopathy is a rare, serious adverse reaction initially ...
-
6 ADVERSE REACTIONS The following serious or otherwise important adverse reactions are described elsewhere in the labeling: • Fibrosing Colonopathy [see Warnings and Precautions (5.1)] • Irritation of the Oral ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Published data from case reports with pancrelipase use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or other ...
-
10 OVERDOSAGE Chronic high dosages of pancreatic enzyme products have been associated with fibrosing colonopathy and colonic strictures [see Warnings and Precautions (5.1)]. High dosages of pancreatic enzyme ...
-
11 DESCRIPTION Pancrelipase is a pancreatic enzyme product consisting of a mixture of enzymes including lipases, proteases, and amylases, and is an extract derived from porcine pancreatic glands. VIOKACE ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Pancreatic enzyme products contain a mixture of lipases, proteases, and amylases that catalyze the hydrolysis of fats to monoglycerides, glycerol and free fatty acids ...
-
14 CLINICAL STUDIES A randomized, double-blind, placebo-controlled, parallel group study was conducted in 50 adult patients, aged 24 to 70 years, with exocrine pancreatic insufficiency due to chronic pancreatitis or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING VIOKACE (pancrelipase) tablets are supplied as follows: Strength - Description - Supplied As - NDC Number - 10,440 USP units of lipase; 39,150 USP units of protease; 39,150 USP ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide). Fibrosing Colonopathy - Advise patients that if signs and symptoms of colon stricture formation occur ...
-
Manufactured by: Viokace, LLC - 1007 US Highway 202/206, Bridgewater, NJ 08807, USA - US License No. 2196 - Manufactured for: Aimmune Therapeutics, Inc. Bridgewater, NJ 08807, USA - ©2024 Nestlé. All ...
-
Medication Guide MEDICATION GUIDE - VIOKACE ® (vye-oh-kase) (pancrelipase) Tablets, for oral use - What is the most important information I should know about VIOKACE? VIOKACE may increase your chance of ...
-
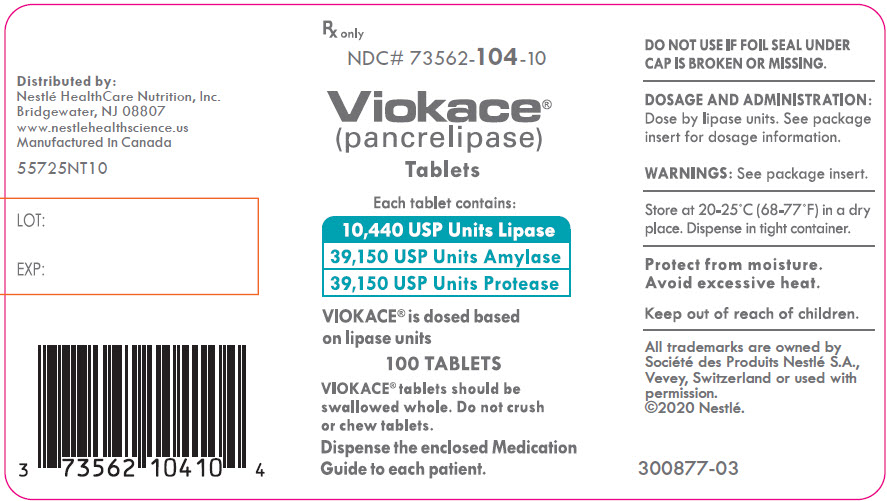
PRINCIPAL DISPLAY PANEL - Lipase 10,440 USP Units Bottle Label NDC 73562-104-10 - Rx only - NDC# 73562-104-10 - Viokace® (pancrelipase) Tablets - Each tablet contains: 10,440 USP Units Lipase - 39,150 USP Units Amylase - 39,150 USP Units ...
-
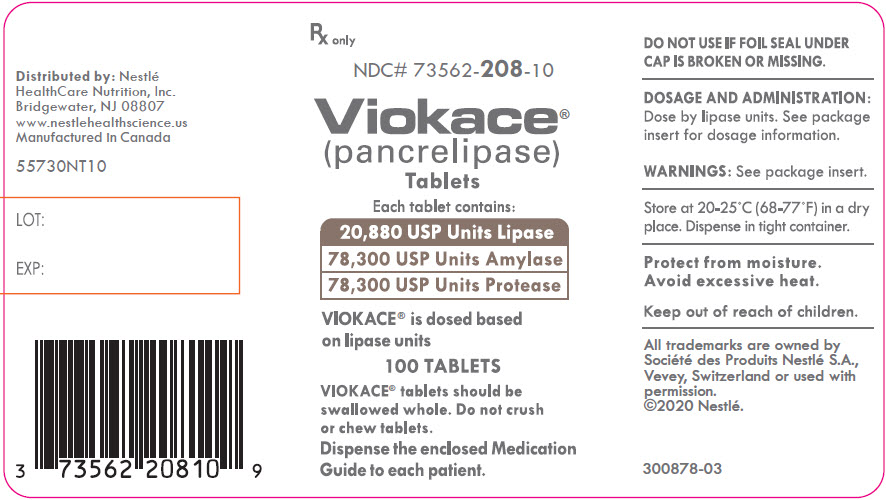
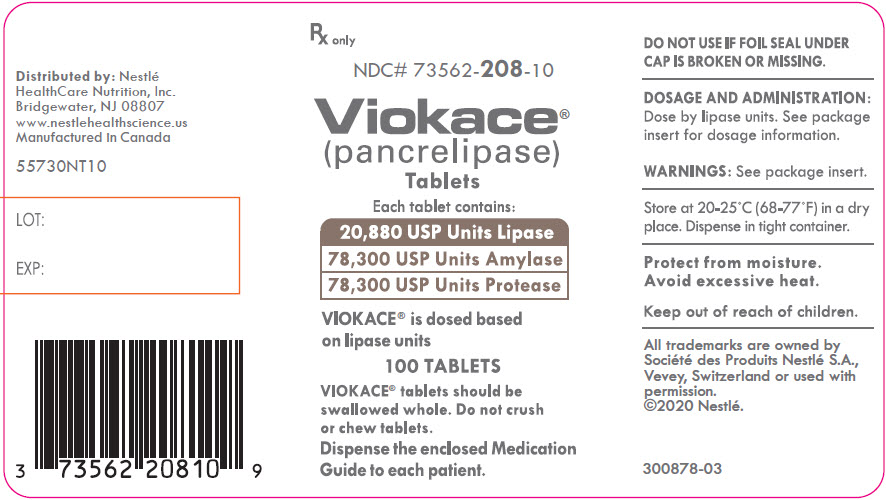
PRINCIPAL DISPLAY PANEL - Lipase 20,880 USP Units Bottle Label NDC 73562-208-10 - Rx only - NDC# 73562-208-10 - Viokace® (pancrelipase) Tablets - Each tablet contains: 20,880 USP Units Lipase - 78,300 USP Units Amylase - 78,330 USP Units ...
-
INGREDIENTS AND APPEARANCEProduct Information