Label: VIADOX- dexamethasone sodium phosphate injection
-
Contains inactivated NDC Code(s)
NDC Code(s): 70529-045-01, 70529-045-02, 70529-045-05 - Packager: IT3 Medical LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
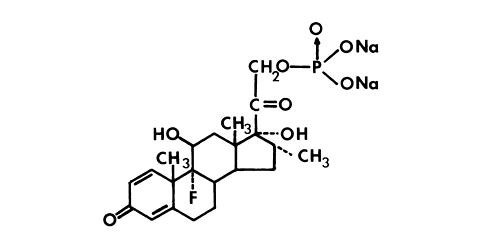
DESCRIPTION
Dexamethasone sodium phosphate injection, USP is a water-soluble inorganic ester of dexamethasone which produces a rapid response even when injected intramuscularly. Dexamethasone sodium ...
-
ACTIONS
Naturally occurring glucocorticoids (hydrocortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are ...
-
INDICATIONS
A. Intravenous or intramuscular administration. When oral therapy is not feasible and the strength, dosage form, and route of administration of the drug reasonably lend the preparation to the ...
-
CONTRAINDICATIONS
Systemic fungal infections.
-
WARNINGS
Serious Neurologic Adverse Reactions with Epidural Administration - Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids. Specific ...
-
PRECAUTIONS
Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy ...
-
ADVERSE REACTIONS
Fluid and electrolyte disturbances: Sodium retention - Fluid retention - Congestive heart failure in susceptible patients - Potassium loss - Hypokalemic alkalosis - Hypertension - Musculoskeletal: Muscle ...
-
DOSAGE AND ADMINISTRATION
A. Intravenous or intramuscular administration. The initial dosage of Dexamethasone Sodium Phosphate Injection may vary from 0.50 mg/day to 9.0 mg/day depending on the specific disease entity ...
-
HOW SUPPLIED
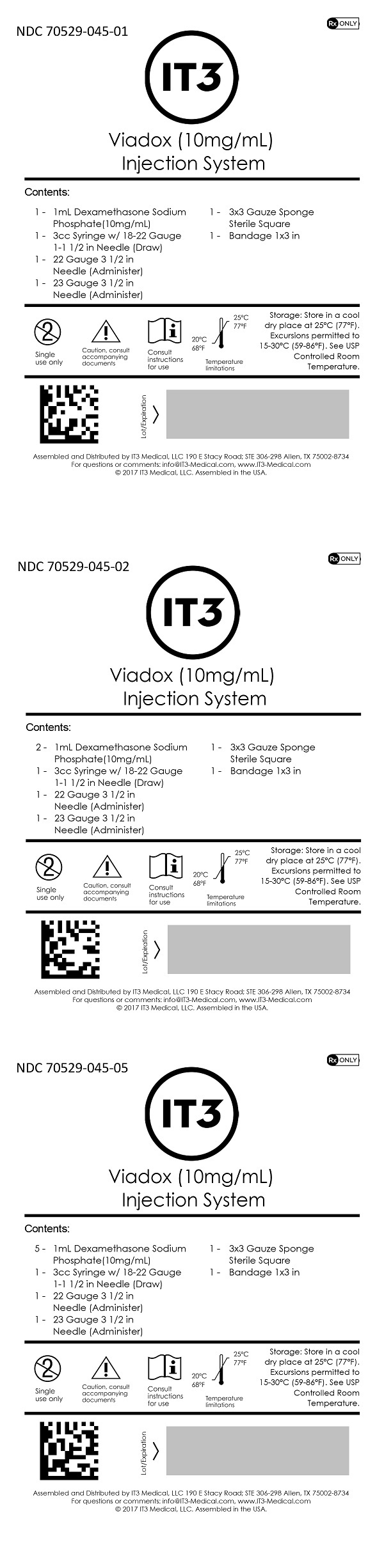
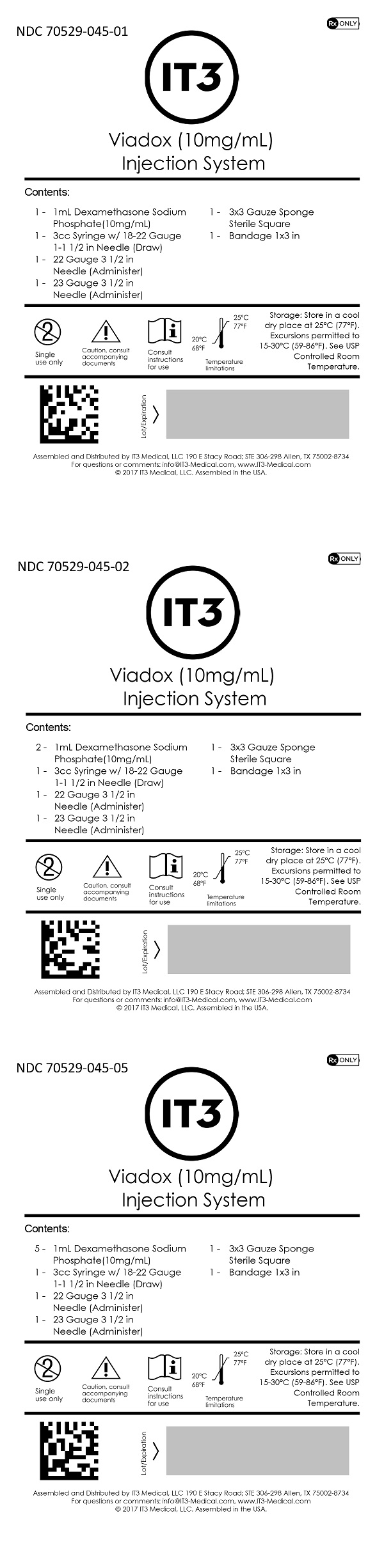
Dexamethasone Sodium Phosphate Injection, USP is available in the following package: 10 mg/mL -1 mL vial
-
StorageStore in a cool dry place at 25°C (77°F). Excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
-
SPL UNCLASSIFIED SECTIONContents (NDC 70529-045-01): 1 - 1mL Dexamethasone Sodium Phosphate (10mg/mL) 1 - 3cc Syringe w/ 18-22 Gauge 1-1 1/2 in Needle (Draw) 1 - 22 Gauge 3 1/2 in Needle (Administer) 1 - 23 Gauge 3 1/2 ...
-
SPL UNCLASSIFIED SECTIONAssembled and Distributed by IT3 Medical, LLC - 190 E Stacy Road; STE 306-298 Allen, TX 75002-8734 - For questions or comments: info@IT3-Medical.com, www.IT3-Medical.com
-
Packaging
-
INGREDIENTS AND APPEARANCEProduct Information