Label: MEPSEVII- vestronidase alfa injection
- NDC Code(s): 69794-001-01
- Packager: Ultragenyx Pharmaceutical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 10, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MEPSEVII safely and effectively. See full prescribing information for MEPSEVII. MEPSEVII® (vestronidase alfa-vjbk) injection ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ANAPHYLAXIS
-
Anaphylaxis has occurred with MEPSEVII administration, as early as the first dose [see Warnings and Precautions (5.1)], therefore appropriate medical support should be readily available when MEPSEVII is administered.
-
Closely observe patients during and for 60 minutes after MEPSEVII infusion [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
- Immediately discontinue the MEPSEVII infusion if the patient experiences anaphylaxis [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
-
Anaphylaxis has occurred with MEPSEVII administration, as early as the first dose [see Warnings and Precautions (5.1)], therefore appropriate medical support should be readily available when MEPSEVII is administered.
-
1 INDICATIONS AND USAGE
MEPSEVII is indicated in pediatric and adult patients for the treatment of Mucopolysaccharidosis VII (MPS VII, Sly syndrome). Limitations of Use - The effect of MEPSEVII on the central nervous ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - MEPSEVII should be administered under the supervision of a healthcare professional with the capability to manage anaphylaxis. Premedication is recommended 30 to 60 ...
-
3 DOSAGE FORMS AND STRENGTHS
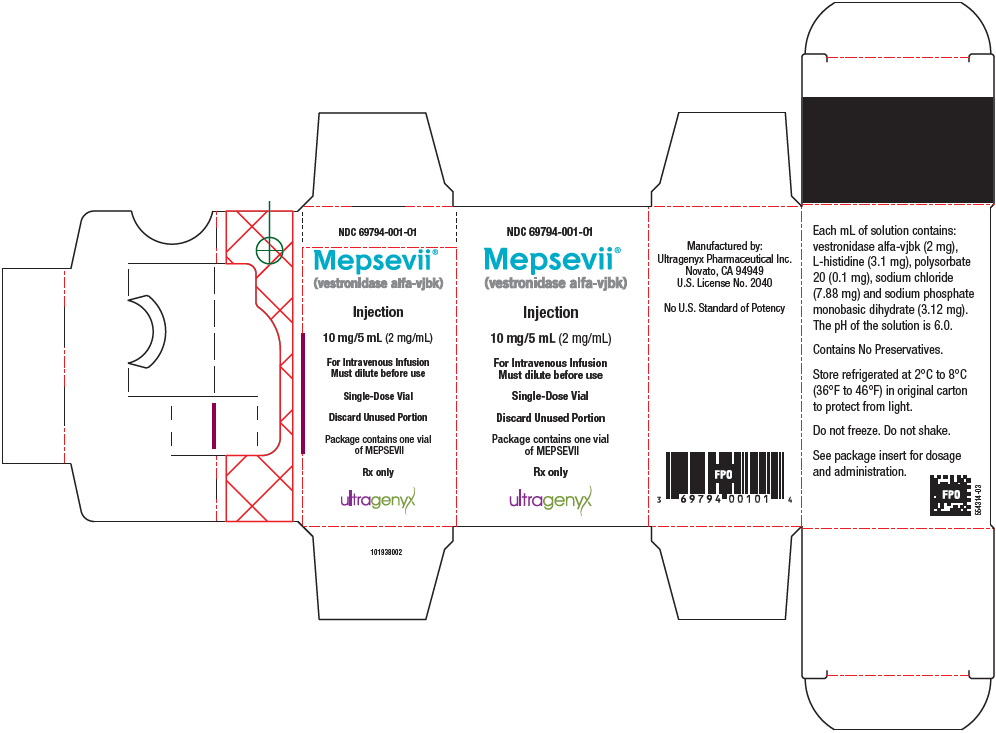
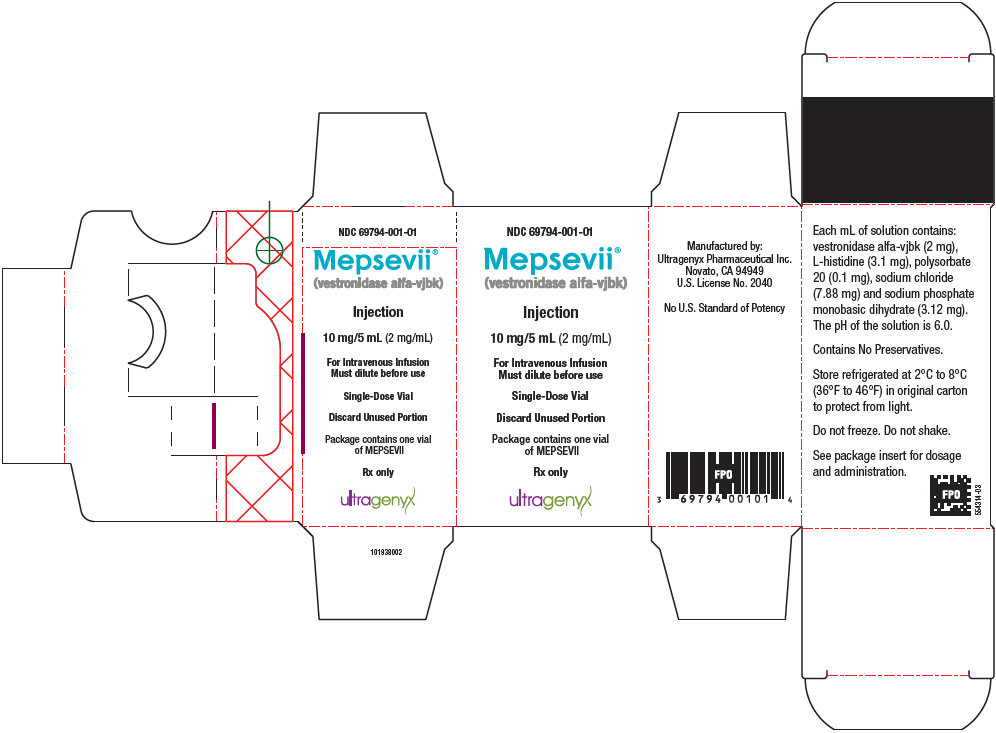
Injection: 10 mg/5 mL (2 mg/mL) as a colorless to slightly yellow liquid in a single-dose vial.
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis - Anaphylaxis to MEPSEVII was reported in 2 of 20 patients in the clinical program [see Adverse Reactions (6.1)]. These reactions occurred during MEPSEVII infusion and were ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling: Anaphylaxis [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because clinical ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - There are no available data on MEPSEVII use in pregnant women to determine a drug-associated risk of adverse developmental outcomes. In embryofetal development ...
-
11 DESCRIPTION
Vestronidase alfa-vjbk is a recombinant human lysosomal beta glucuronidase which is a purified human enzyme produced by recombinant DNA technology in a Chinese hamster ovary cell line. Purified ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Mucopolysaccharidosis VII (MPS VII or Sly syndrome) is a lysosomal disorder characterized by the deficiency of GUS that results in GAG accumulation in cells ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate the mutagenic potential have not been ...
-
14 CLINICAL STUDIES
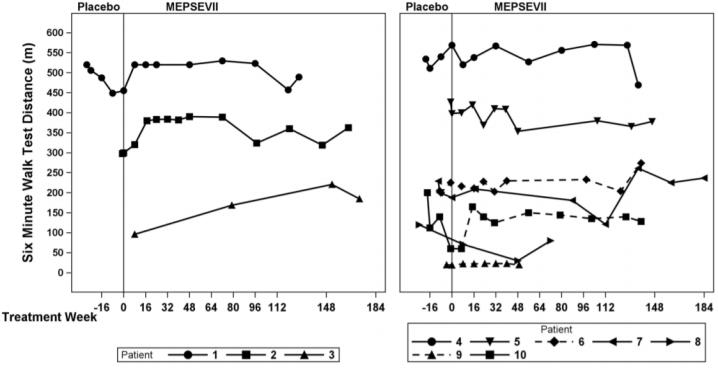
The clinical program for MEPSEVII included 23 patients with MPS VII, 17 of whom were evaluable for efficacy, 20 for safety, and 23 for immunogenicity. Patients were enrolled in clinical trials and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
MEPSEVII (vestronidase alfa-vjbk) injection is a colorless to slightly yellow liquid supplied as a carton containing one 10 mg/5 mL (2 mg/mL) single-dose vial (NDC 69794-001-01). Store under ...
-
17 PATIENT COUNSELING INFORMATION
Anaphylaxis - Advise patients and caregivers that anaphylaxis has occurred with MEPSEVII administration. Inform patients of the signs and symptoms of anaphylaxis, and have them seek immediate ...
-
PRINCIPAL DISPLAY PANEL - 10 mg/5 mL Vial CartonNDC 69794-001-01 - Mepsevii® (vestronidase alfa-vjbk) Injection - 10 mg/5 mL (2 mg/mL) For Intravenous Infusion - Must dilute before use - Single-Dose Vial - Discard Unused Portion - Package contains one ...
-
INGREDIENTS AND APPEARANCEProduct Information