Label: VENIPUNCTURE PX1- lidocaine hydrochloride kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 17478-840-05, 67777-121-13, 70529-254-01 - Packager: IT3 Medical LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
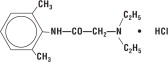
DESCRIPTIONLidocaine HCl 2% Jelly is a sterile, aqueous product that contains a local anesthetic agent and is administered topically. (See INDICATIONS for specific uses.) Lidocaine HCl 2% Jelly contains ...
-
CLINICAL PHARMACOLOGYMechanism of Action: Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic ...
-
INDICATIONS AND USAGELidocaine HCl 2% Jelly is indicated for prevention and control of pain in procedures involving the male and female urethra, for topical treatment of painful urethritis, and as an anesthetic ...
-
CONTRAINDICATIONSLidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to other components of Lidocaine HCl 2% Jelly.
-
WARNINGSEXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS. PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ...
-
PRECAUTIONSGeneral: The safety and effectiveness of lidocaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. (See WARNINGS and ADVERSE REACTIONS.) The ...
-
ADVERSE REACTIONSAdverse experiences following the administration of lidocaine are similar in nature to those observed in other amide local anesthetic agents. These adverse experiences are, in general ...
-
OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics. (See ADVERSE REACTIONS, WARNINGS, and ...
-
DOSAGE AND ADMINISTRATIONWhen Lidocaine HCl 2% Jelly is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind. The dosage varies and depends upon ...
-
MAXIMUM DOSAGENo more than 600 mg of lidocaine HCl should be given in any 12 hour period. Children: It is difficult to recommend a maximum dosage of any drug for children since this varies as a function of ...
-
HOW SUPPLIEDLidocaine HCI 2% Jelly is supplied in the listed dosage forms. NDC 17478-840-30 30 mL aluminum tube - NDC 17478-840-05 5 mL aluminum tube - A detachable ...
- Sterile Alcohol Prep Pads
-
ACTIVE INGREDIENTActive Ingredient Purpose - Isopropyl Alcohol 70% v/v Antiseptic
-
Use: alcohol_prepFor preparation of the skin prior to injection.
-
Warnings:For external use only - Flammable, keep away from flame or fire - Not for use with electrocautinary devices or procedures - Do not use in eyes - Sterile unless package is damaged or open.
-
Indications and Usage:Stop use and ask a doctor if: Irritation or redness develops - condition persists for more than 72 hours - Cleansing of an injection site
-
Keep out of reach of children.In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
-
Directions:Wipe injection site vigorously and discard
-
Other information:Store at room temperature: 15 deg C to 30 deg C 59 deg F to 86 deg F - avoid excessive heat
-
Inactive IngredientInactive Ingredient - Water
-
Venipuncture Px1Phlebotomy System
(W/ 2 % Lidocaine Hydrochloride Jelly, USP- 5.0 mL) Contents - 1- Bio-Hazard Specimen Transport Bag - 1-Pair of Sterile Vinyl Exam Gloves (Medium), Latex Free - 2-Sheer Adhesive Bandages, Spots - 1- Basic conforming Stretch Gauze ...
-
Packaging-Kit Label

-
Packaging- Kit Components Labeling
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information