Label: VELPHORO- sucroferric oxyhydroxide tablet, chewable
- NDC Code(s): 49230-645-51
- Packager: Fresenius Medical Care North America
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VELPHORO - ®safely and effectively. See full prescribing information for VELPHORO - ®. VELPHORO - ®(sucroferric oxyhydroxide ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVelphoro is indicated for the control of serum phosphorus levels in adults and pediatric patients 9 years of age and older with chronic kidney disease (CKD) on dialysis.
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - The recommended starting dose of Velphoro in adults and pediatric patients 12 years of age and older is one 500 mg tablet three times daily with meals. The ...
-
3 DOSAGE FORMS AND STRENGTHSVelphoro is supplied in a strength of 500 mg as a brown, circular, bi-planar chewable tablet embossed with “PA 500” on one side. Each chewable tablet contains 500 mg iron (equivalent to 2,500 mg ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Monitoring in Patients with Gastrointestinal Disorders or Iron Accumulation Disorders - Each chewable tablet contains 500 mg iron (equivalent to 2,500 mg sucroferric oxyhydroxide) [see ...
-
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - Adult Patients - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot ...
-
7 DRUG INTERACTIONSTable 1 Oral drugs that can be administered concomitantly with Velphoro - Calcitriol - Ciprofloxacin - Digoxin - Enalapril - Furosemide - HMG-CoA ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Velphoro is not systemically absorbed following oral administration and maternal use is not expected to result in fetal exposure to the drug. Data - Animal Data - In ...
-
10 OVERDOSAGEThere are no reports of overdosage with Velphoro in patients. Since the absorption of iron from Velphoro is low - [see Clinical Pharmacology ( 12.3)] , the risk of systemic iron ...
-
11 DESCRIPTIONThe Velphoro drug substance is a mixture of polynuclear iron(III)-oxyhydroxide, sucrose, and starches. The active moiety of Velphoro, polynuclear iron(III)-oxyhydroxide, is practically insoluble ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - In the aqueous environment of the GI tract, phosphate binding takes place by ligand exchange between hydroxyl groups and/or water in sucroferric oxyhydroxide and the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies were performed in mice and rats. In the 2-year carcinogenicity study in mice, animals were given Velphoro by ...
-
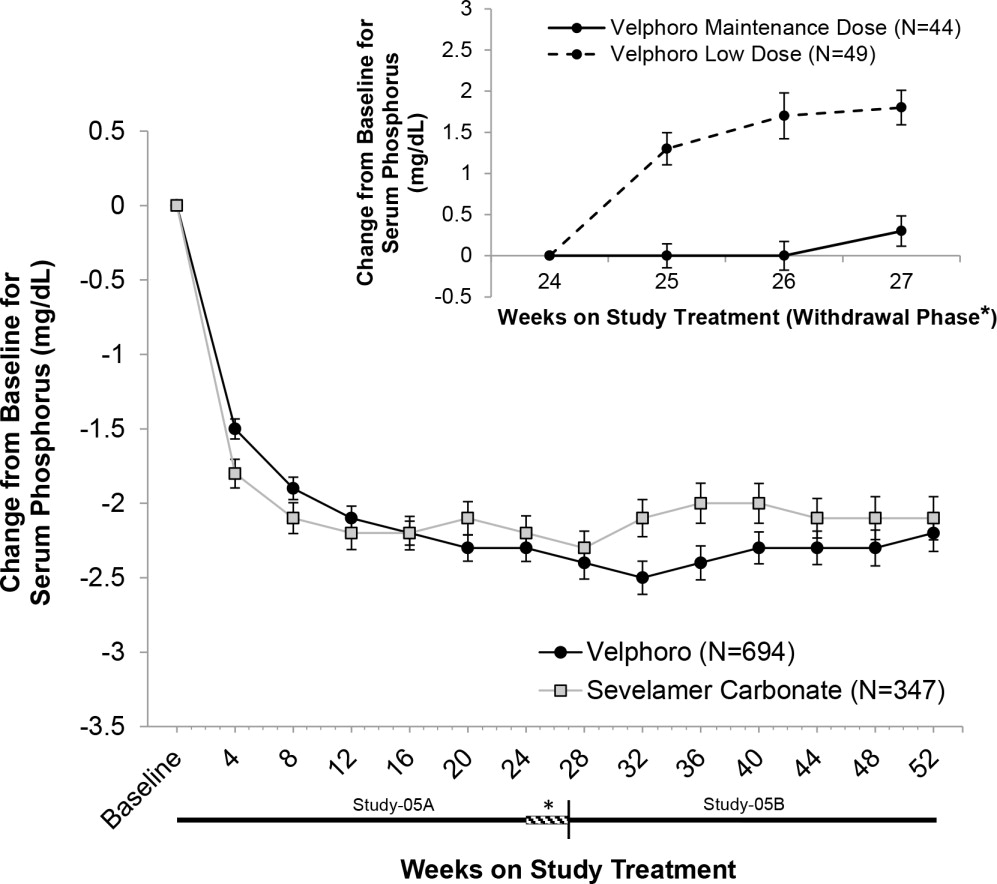
14 CLINICAL STUDIES14.1 Fixed-dose Study - In Study-03A, 154 ESRD adult patients on hemodialysis who were hyperphosphatemic (serum phosphorus >5.5 mg/dL but <7.75 mg/dL) following a 2-week phosphate binder washout ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Velphoro chewable tablets are brown, circular, bi-planar tablets, embossed with “PA 500” on 1 side. Each tablet of Velphoro contains 500 mg iron as sucroferric oxyhydroxide ...
-
17 PATIENT COUNSELING INFORMATIONInform patients that Velphoro tablets should be chewed or crushed. Do not swallow whole - [see Dosage and Administration ( 2.2)]. Advise patients that Velphoro should be taken with ...
-
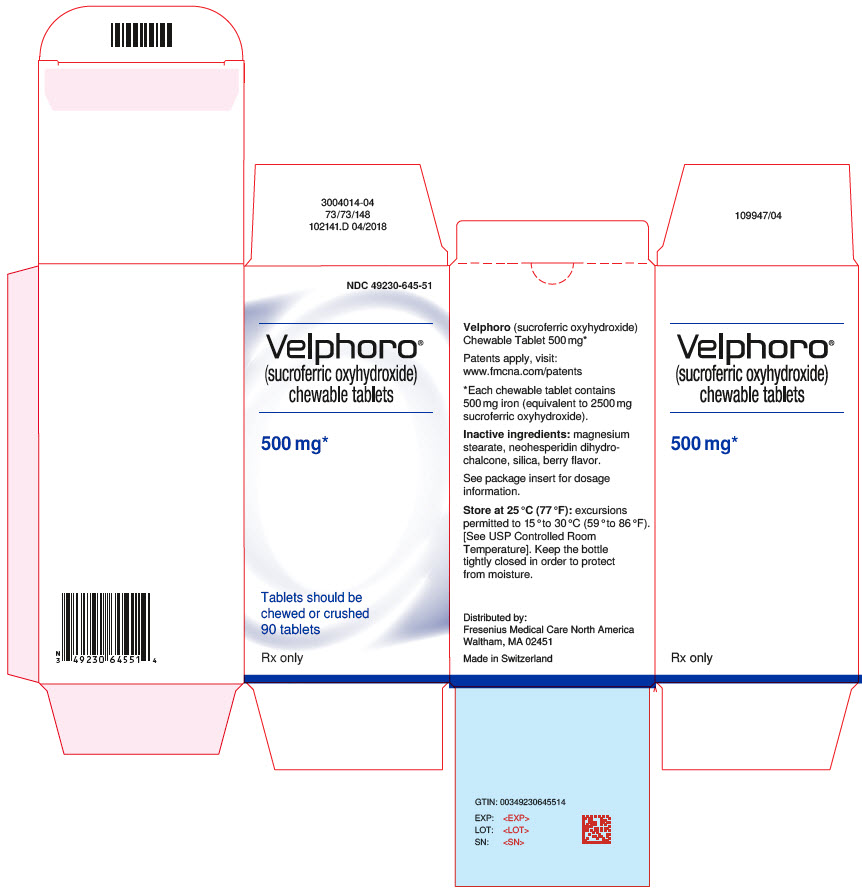
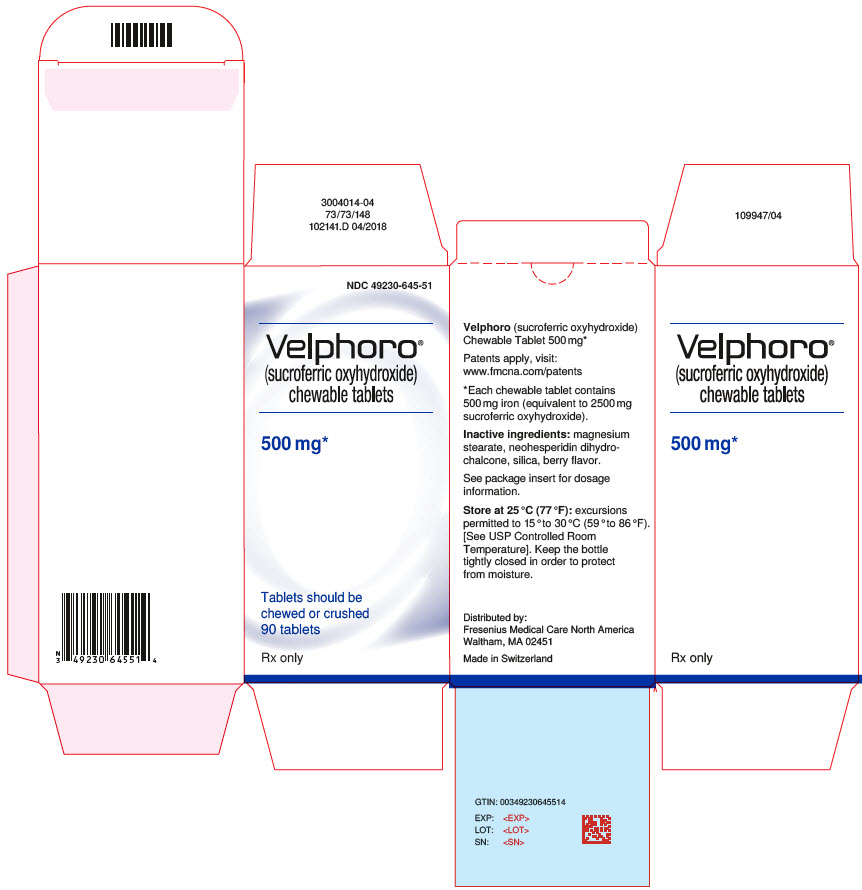
PRINCIPAL DISPLAY PANELPrincipal Display Label - Bottle Label - NDC 49230-645-51
-
PRINCIPAL DISPLAY PANELPrincipal Display Label - Bottle Carton - NDC 49230-645-51
-
INGREDIENTS AND APPEARANCEProduct Information