Label: VECTICAL- calcitriol ointment
- NDC Code(s): 0299-2012-05, 0299-2012-10
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VECTICAL Ointment safely and effectively. See full prescribing information for VECTICAL Ointment. VECTICAL Ointment, for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indication - VECTICAL Ointment is indicated for the topical treatment of mild to moderate plaque psoriasis in adults and pediatric patients 2 years and older. 1.2 Limitations of ...
-
2 DOSAGE AND ADMINISTRATIONApply VECTICAL Ointment to affected areas twice daily, morning and evening - Adults: • The maximum weekly dose should not exceed 200 grams. Pediatrics: • 2 to 6 years of ...
-
3 DOSAGE FORMS AND STRENGTHSOintment, 3 mcg/g. Each gram of VECTICAL Ointment contains 3 micrograms (mcg/g) of calcitriol.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Effects on Calcium Metabolism - In controlled clinical trials hypercalcemia was observed in subjects exposed to VECTICAL Ointment. If aberrations in parameters of calcium metabolism ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from pregnancies that occurred during the clinical development of VECTICAL Ointment and published case series of oral and intravenous calcitriol ...
-
10 OVERDOSAGETopically applied calcitriol can be absorbed in sufficient amounts to produce systemic effects [ see Warningsand Precautions ( 5.1) ].
-
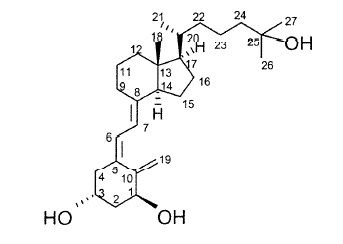
11 DESCRIPTIONVECTICAL (calcitriol) Ointment 3 mcg/g is a vitamin D analog intended for topical application to the skin. The chemical name of the active ingredient is ...
-
12 CLINICAL PHARMACOLOGYThe contribution to efficacy of individual components of the vehicle has not been established. 12.1 Mechanism of Action - The mechanism of action of calcitriol in the treatment of psoriasis has ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - When calcitriol was applied topically to mice for up to 24 months, no significant changes in tumor incidence were observed ...
-
14 CLINICAL STUDIESIn two, multicenter, double-blind, vehicle-controlled studies, a total of 839 subjects with psoriasis rated "mild" or "moderate" using an investigator global assessment scale were treated twice ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - VECTICAL Ointment 3 mcg/g is available in collapsible aluminum tubes of the following package sizes: 100 g tube (NDC 0299-2012-10) 16.2 Storage - Store at Controlled ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Patients using VECTICAL Ointment should receive the following information: This medication is to be used ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - VECTICAL® (Vek te kal) (calcitriol) Ointment - Important: VECTICAL Ointment is for use on the skin only (topical use).Do not use VECTICAL Ointment in ...
- PATIENT MEDICATION INFORMATION
-
PACKAGE LABEL - 100 g CARTONVectical® (calcitriol) Ointment 3 mcg/g - FOR TOPICAL USE ONLY - NDC 0299-2012-10 - Rx Only - NET WT. 100 g - GALDERMA - For topical use only ...
-
INGREDIENTS AND APPEARANCEProduct Information