Label: VARUBI- rolapitant tablet
- NDC Code(s): 70720-101-02
- Packager: TerSera Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VARUBI safely and effectively. See full prescribing information for VARUBI. VARUBI® (rolapitant) tablets, for oral use - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
VARUBI® is indicated in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer ...
-
2 DOSAGE AND ADMINISTRATION
The recommended dosage of VARUBI in adults in combination with a 5-HT3 receptor antagonist and dexamethasone for the prevention of nausea and vomiting with emetogenic cancer chemotherapy is shown ...
-
3 DOSAGE FORMS AND STRENGTHS
Tablets: 90 mg rolapitant; film-coated, blue capsule shaped, debossed with T0101 on one side and 100 on the other side.
-
4 CONTRAINDICATIONS
VARUBI is contraindicated in patients taking CYP2D6 substrates with a narrow therapeutic index, such as thioridazine and pimozide. VARUBI can significantly increase the plasma concentrations of ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Interaction with CYP2D6 Substrates - Rolapitant is a moderate inhibitor of CYP2D6. Exposure to dextromethorphan, a CYP2D6 substrate, following a single dose of rolapitant increased about ...
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling: Interaction with CYP2D6 Substrates [see Contraindications (4), Warnings and Precautions ...
-
7 DRUG INTERACTIONS
Rolapitant is a moderate CYP2D6 inhibitor. The inhibition of CYP2D6 persisted on Day 28 with a 2.3-fold increase in dextromethorphan concentrations, the last time point measured. The inhibitory ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - The limited data with VARUBI use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In animal reproduction ...
-
10 OVERDOSAGE
There are no data on overdose with VARUBI. There is no antidote for VARUBI overdose. Discontinue VARUBI in the event of overdose, and institute general supportive measures and close ...
-
11 DESCRIPTION
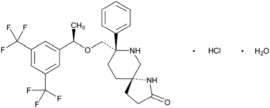
VARUBI contains rolapitant, a substance P/neurokinin 1 (NK1) receptor antagonist. Rolapitant hydrochloride is chemically described as (5S,8S)-8- ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Rolapitant is a selective and competitive antagonist of human substance P/NK1 receptors. Rolapitant does not have significant affinity for the NK2 or NK3 receptors or ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of rolapitant was assessed in 2-year carcinogenicity studies in CD-1 mice and ...
-
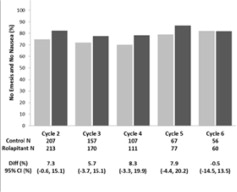
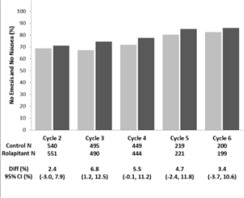
14 CLINICAL STUDIES
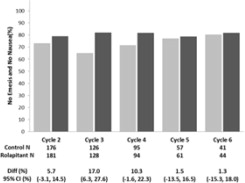
Cisplatin-Based Highly Emetogenic Chemotherapy (HEC) In two multicenter, randomized, double-blind, parallel group, controlled clinical studies (Study 1 and Study 2), the VARUBI regimen ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VARUBI is available as film-coated, capsule shaped, blue tablets, debossed with T0101 on one side and 100 on the other side. Each tablet contains 90 mg rolapitant. VARUBI is packaged in an Aclar ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Drug Interactions - Advise patients to tell their healthcare provider when they start or stop taking any ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 8/2020 - PATIENT INFORMATION - VARUBI® (vuh ROO bee) (rolapitant) tablets, for oral ...
-
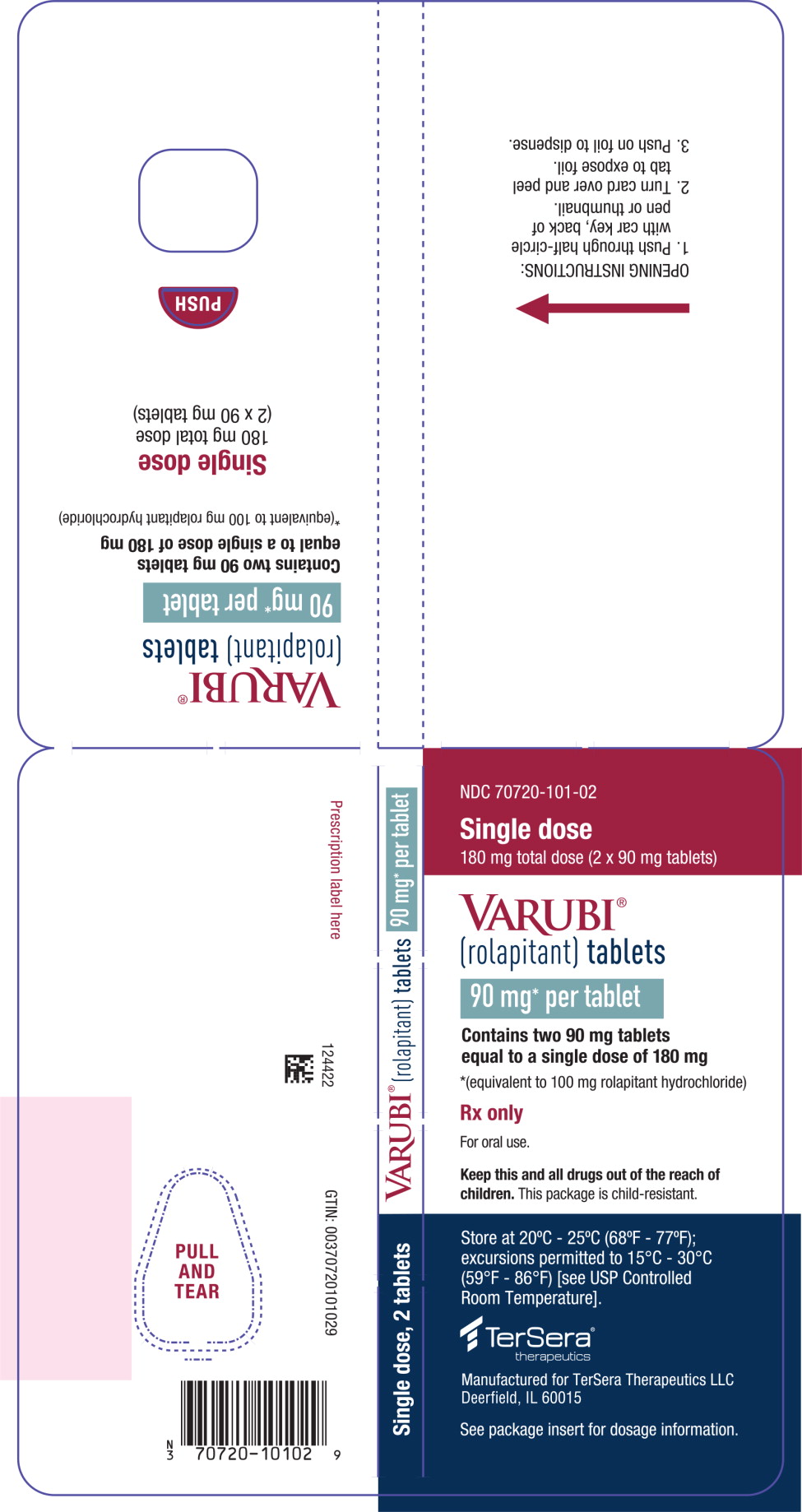
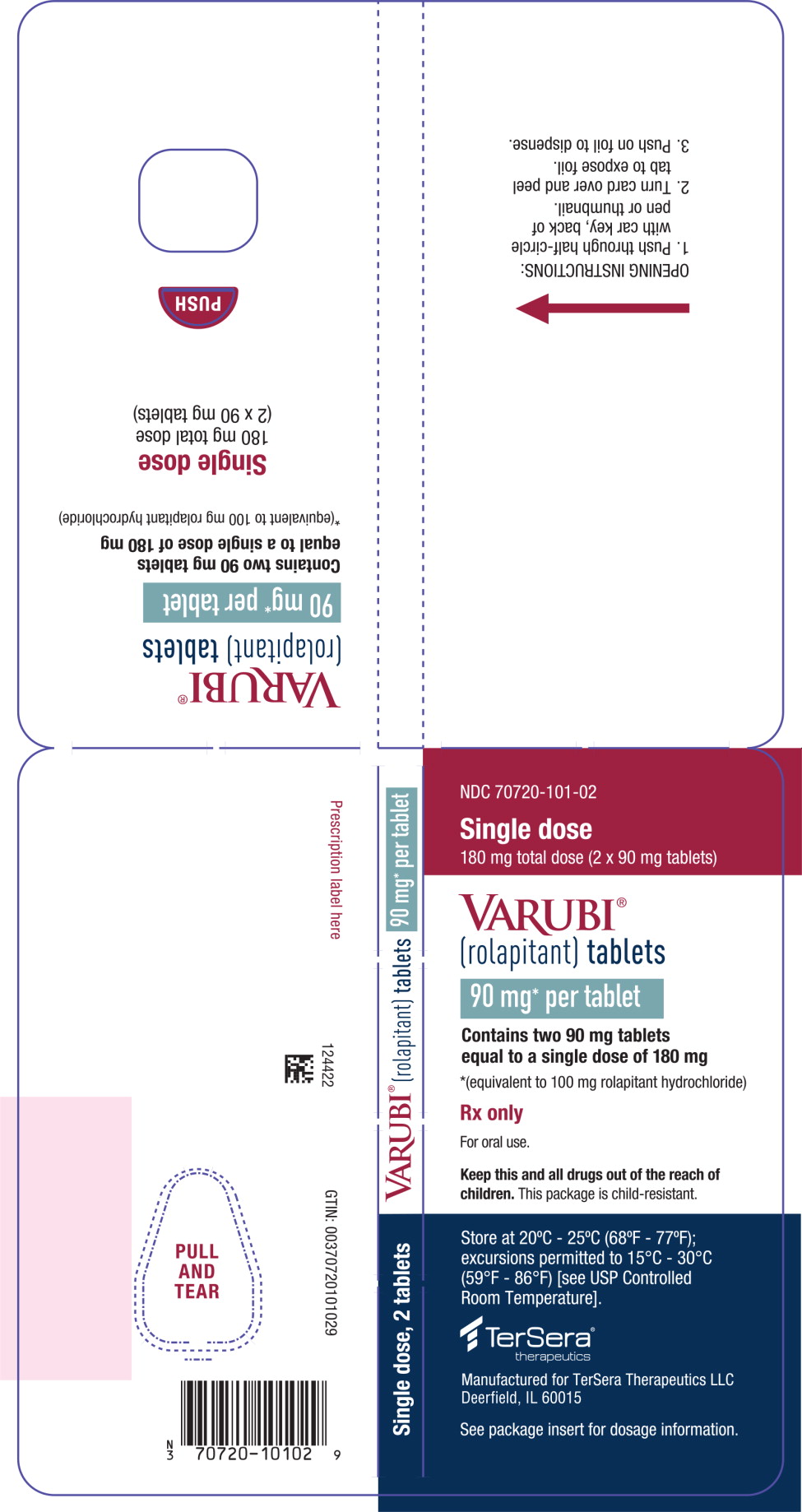
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 90 mg Tablet Blister Wallet - NDC 70720-101-02 - Single dose - 180 mg total dose (2 x 90 mg tablets) VARUBI® (rolapitant) tablets - 90 mg* per tablet - Contains two 90 mg ...
-
PRINCIPAL DISPLAY PANEL - NDC: 70720-101-02 - Blister Backing Label
-
INGREDIENTS AND APPEARANCEProduct Information