Label: VARITHENA- polidocanol kit
- NDC Code(s): 60635-007-01, 60635-018-01, 60635-107-01, 60635-111-01, view more
- Packager: Biocompatibles, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VARITHENA® safely and effectively. See Full Prescribing Information for VARITHENA. VARITHENA (polidocanol injectable foam) ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVARITHENA (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins, and visible varicosities of the great saphenous vein (GSV ...
-
2 DOSAGE AND ADMINISTRATIONFor intravenous use only. VARITHENA is intended for intravenous injection using ultrasound guidance, administered via a single cannula into the lumen of the target incompetent trunk veins or by ...
-
3 DOSAGE FORMS AND STRENGTHSVARITHENA is available in the following presentations: 180 mg/18 mL (10 mg/mL) 77.5 mg/7.75 mL (10 mg/mL) Once activated, VARITHENA is a white, injectable foam delivering a 1% polidocanol ...
-
4 CONTRAINDICATIONSThe use of VARITHENA is contraindicated in patients with: known allergy to polidocanol [see Warnings and Precautions (5.1)] acute thromboembolic disease
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylaxis - Severe allergic reactions have been reported following administration of liquid polidocanol, including anaphylactic reactions, some of them fatal. Observe patients for at least ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under controlled but widely varying conditions, adverse reaction rates observed in clinical trials of VARITHENA cannot be ...
-
7 DRUG INTERACTIONSNo specific drug interaction studies have been performed. There are no known drug interactions with VARITHENA.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Few published case reports with use of polidocanol-containing products, including VARITHENA, in pregnant women have not identified any drug-associated risk for major ...
-
10 OVERDOSAGE There are no known cases of overdosage with VARITHENA. In clinical studies, total volumes of up to 60 mL of VARITHENA per treatment session have been administered.
-
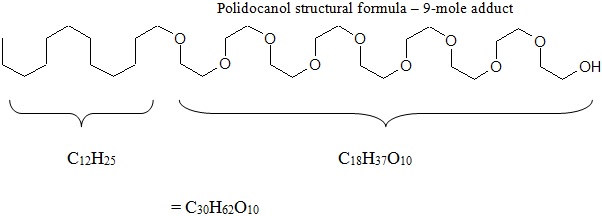
11 DESCRIPTIONVARITHENA injectable foam contains the sclerosant, polidocanol. It is intended for intravenous use only. Chemically, polidocanol is polyoxyl lauryl ether. The structural formula is represented ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - VARITHENA is a drug/device combination product that generates injectable foam. The injectable foam is composed of a liquid and gas phase, both of which are necessary ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential of VARITHENA. No mutagenic activity was ...
-
14 CLINICAL STUDIESVARITHENA was evaluated in two randomized, blinded, multicenter clinical trials designed to assess the efficacy and safety of VARITHENA 0.5%, 1.0%, and 2.0% (VANISH-1) and VARITHENA 0.5% and 1.0 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - VARITHENA (polidocanol injectable foam) product is available in four configurations, each containing two sterile, connected, 303-mL aluminum alloy cylinders, one containing ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to keep post-treatment bandages dry and in place for 48 hours and to wear compression stockings on the treated legs continuously for 2 weeks. Compression stockings should be ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Bi-Canister - NDC 60635-107-01
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Pouch Label - NDC 60635-107-01
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Canister Label - NDC 60635-007-01
-
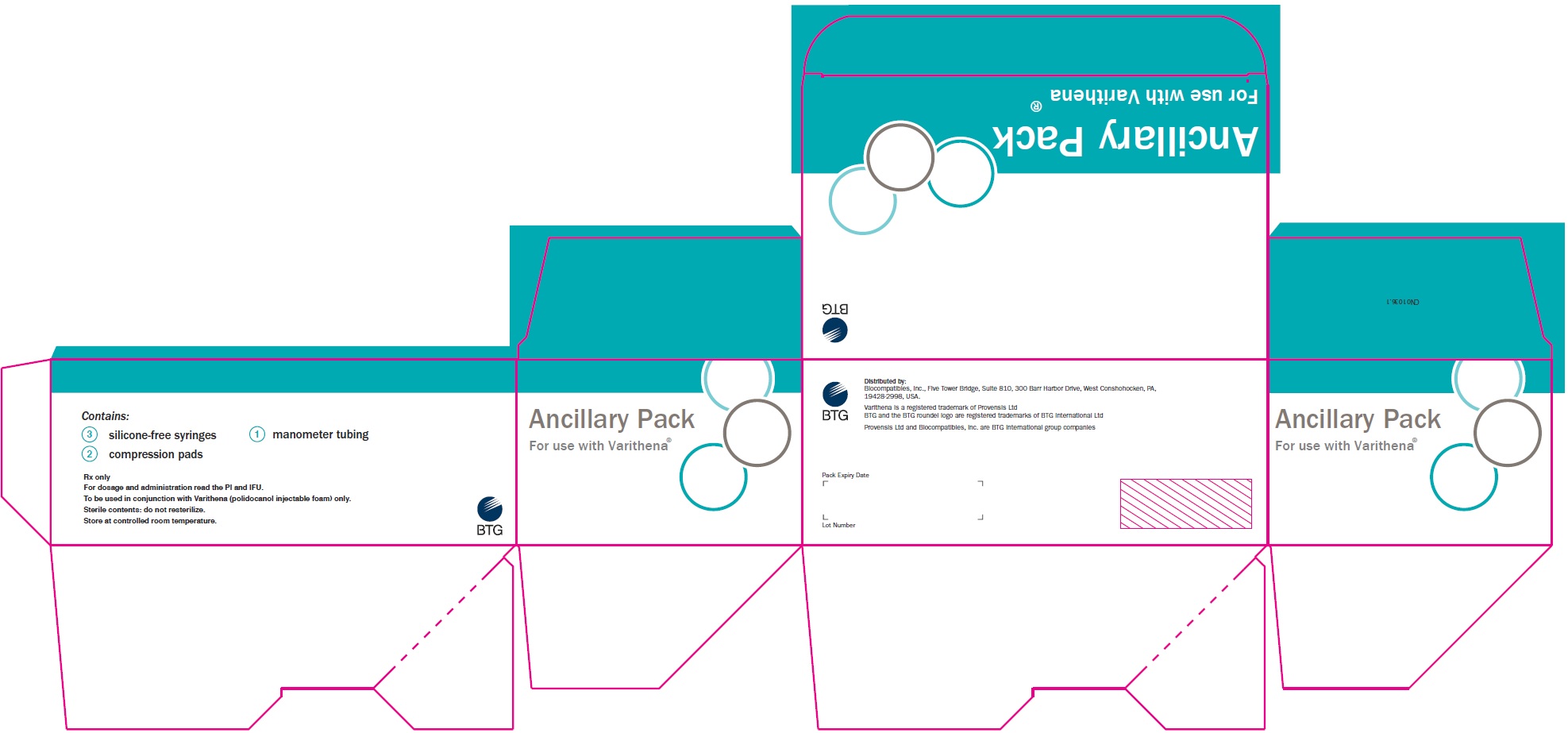
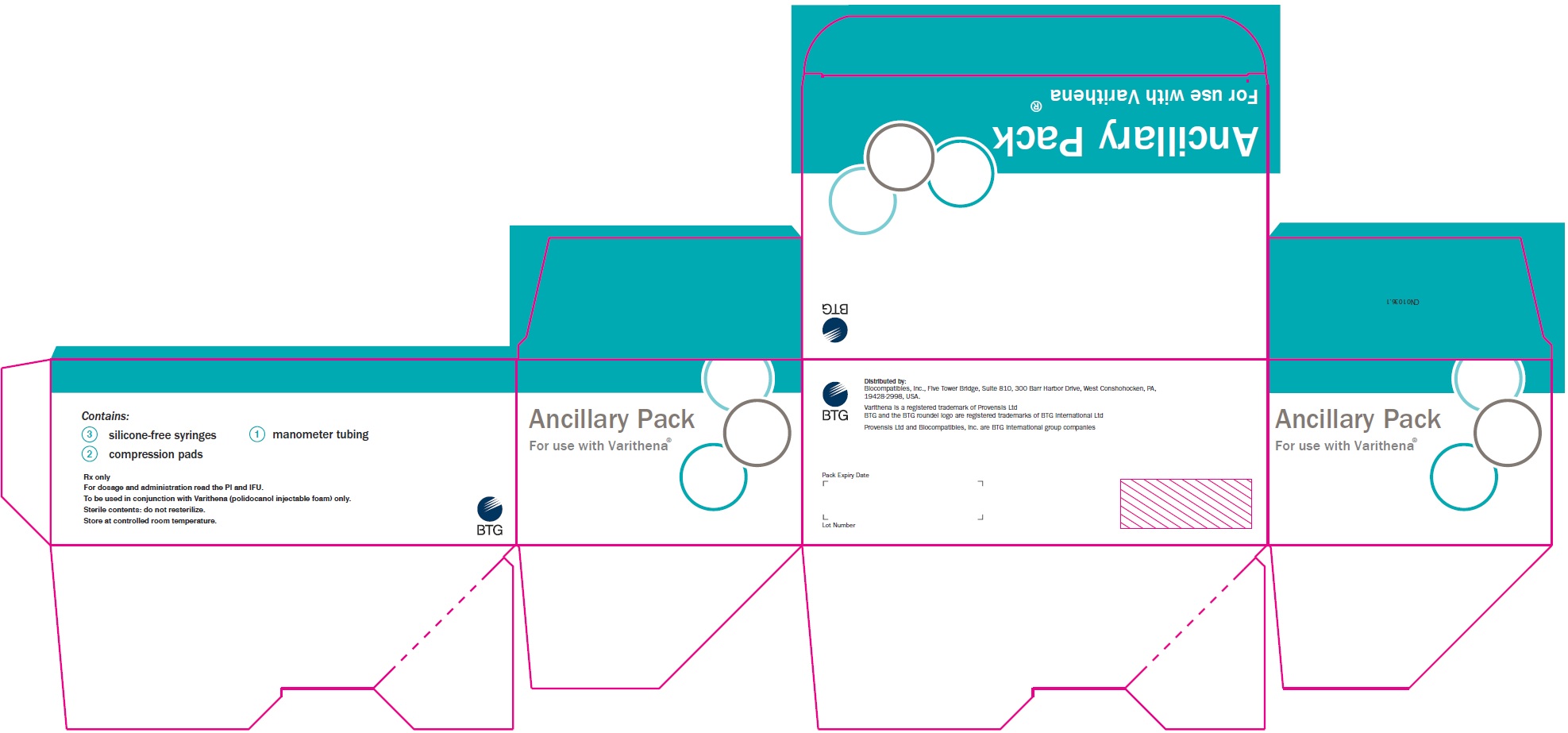
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Ancillary Pack
-
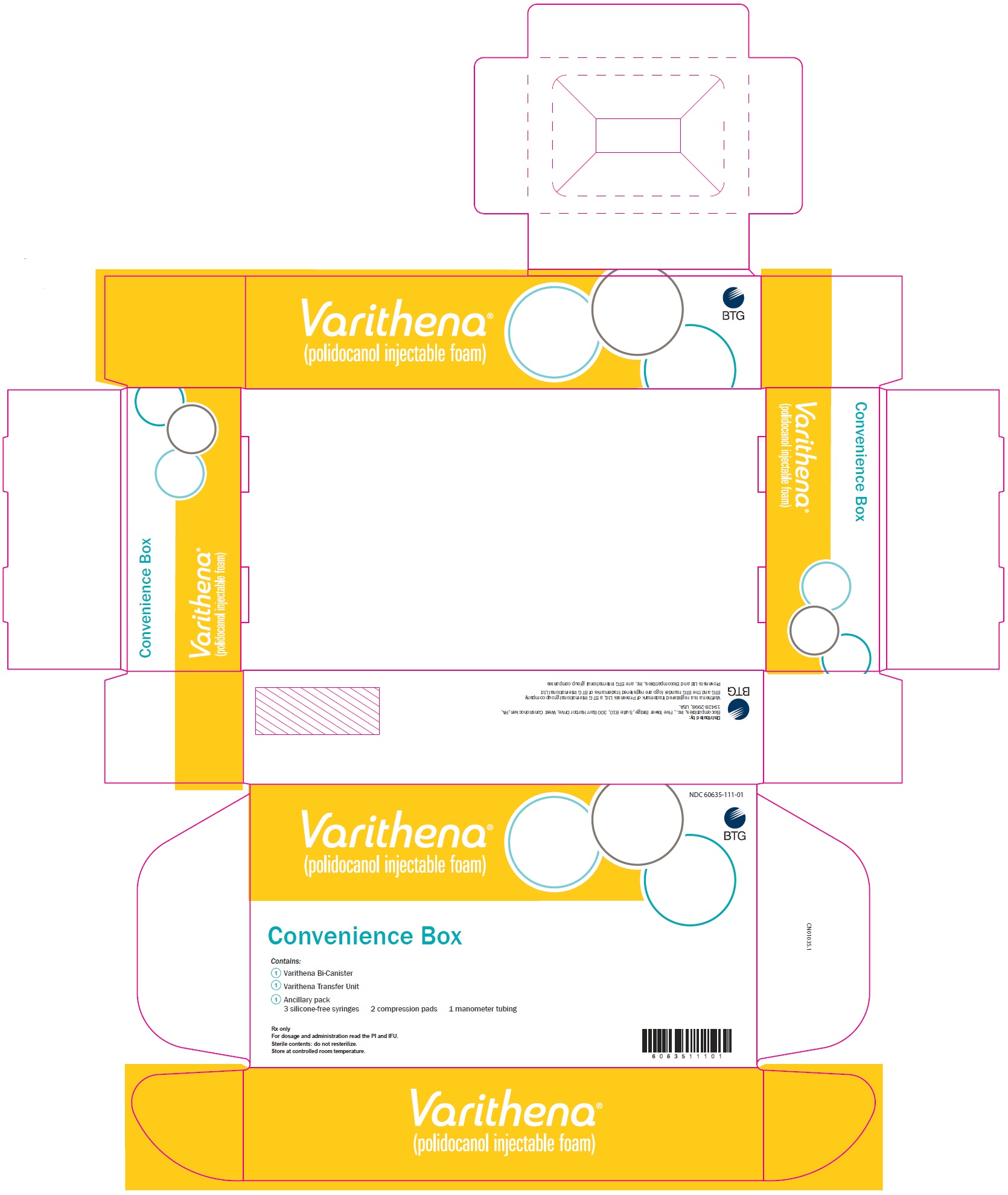
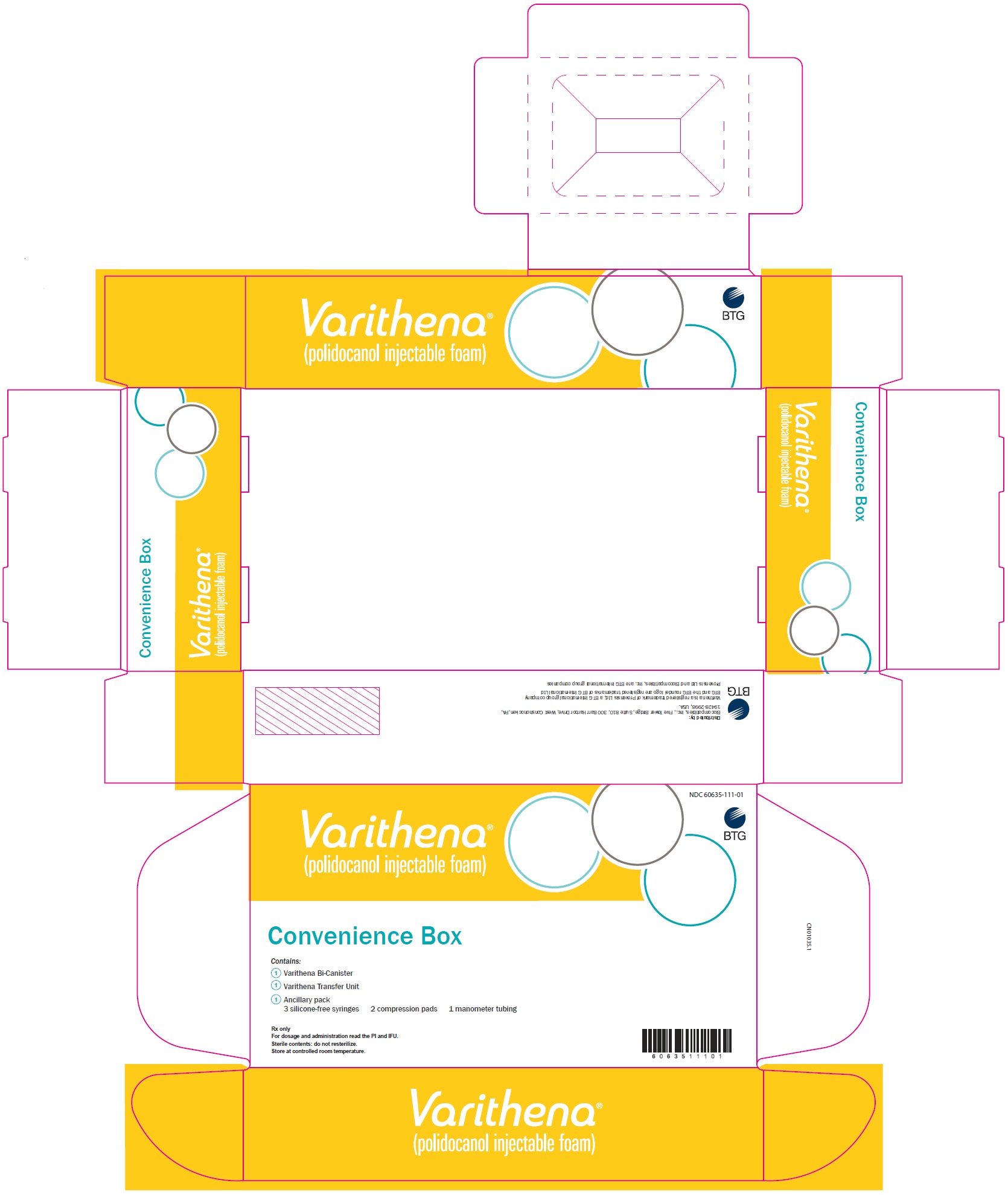
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Convenience Box Carton - NDC 60635-111-01
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Canister Label - NDC 60635-018-01
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Pouch Label - NDC 60635-118-01
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Bi-Canister Box - NDC 60635-118-01
-
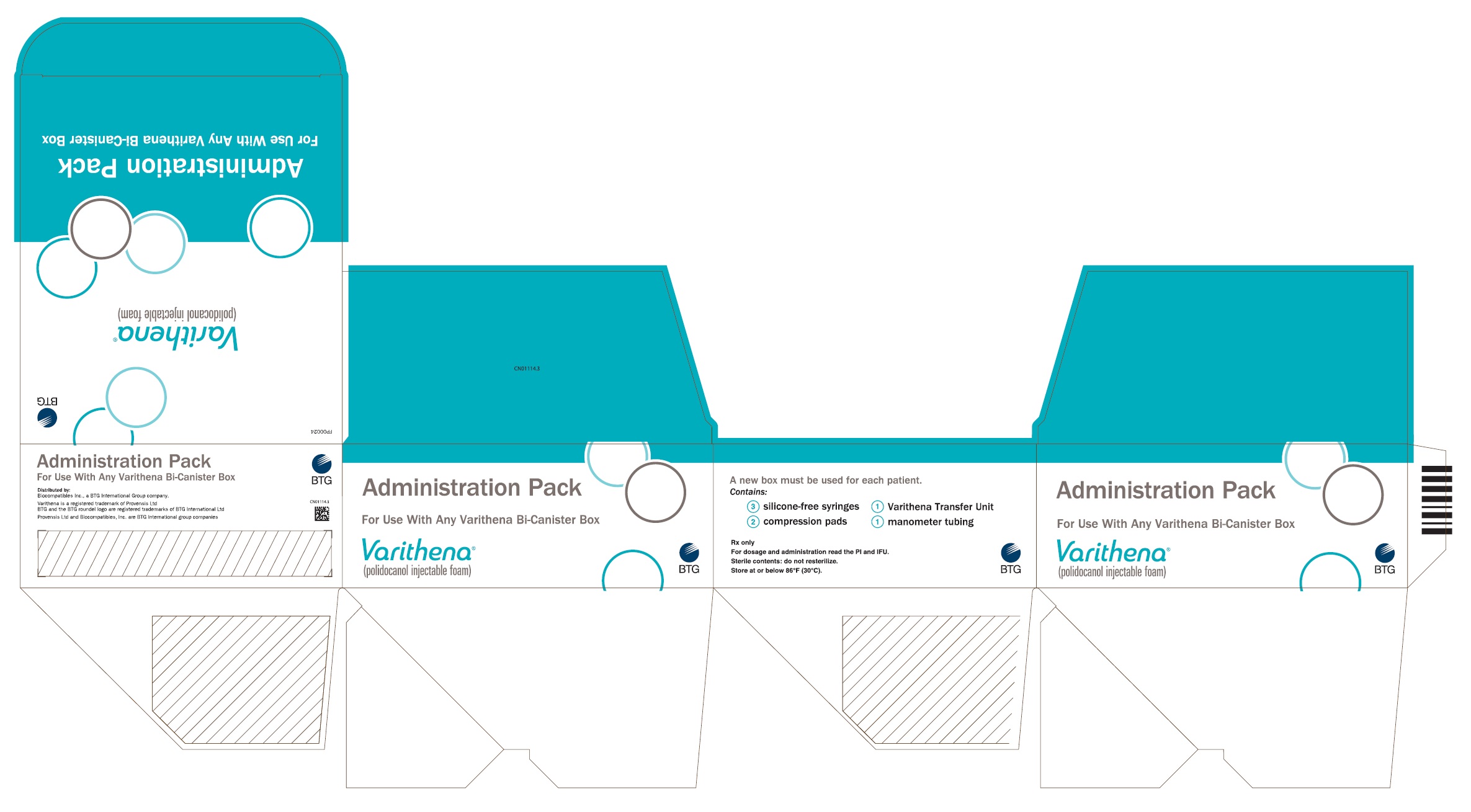
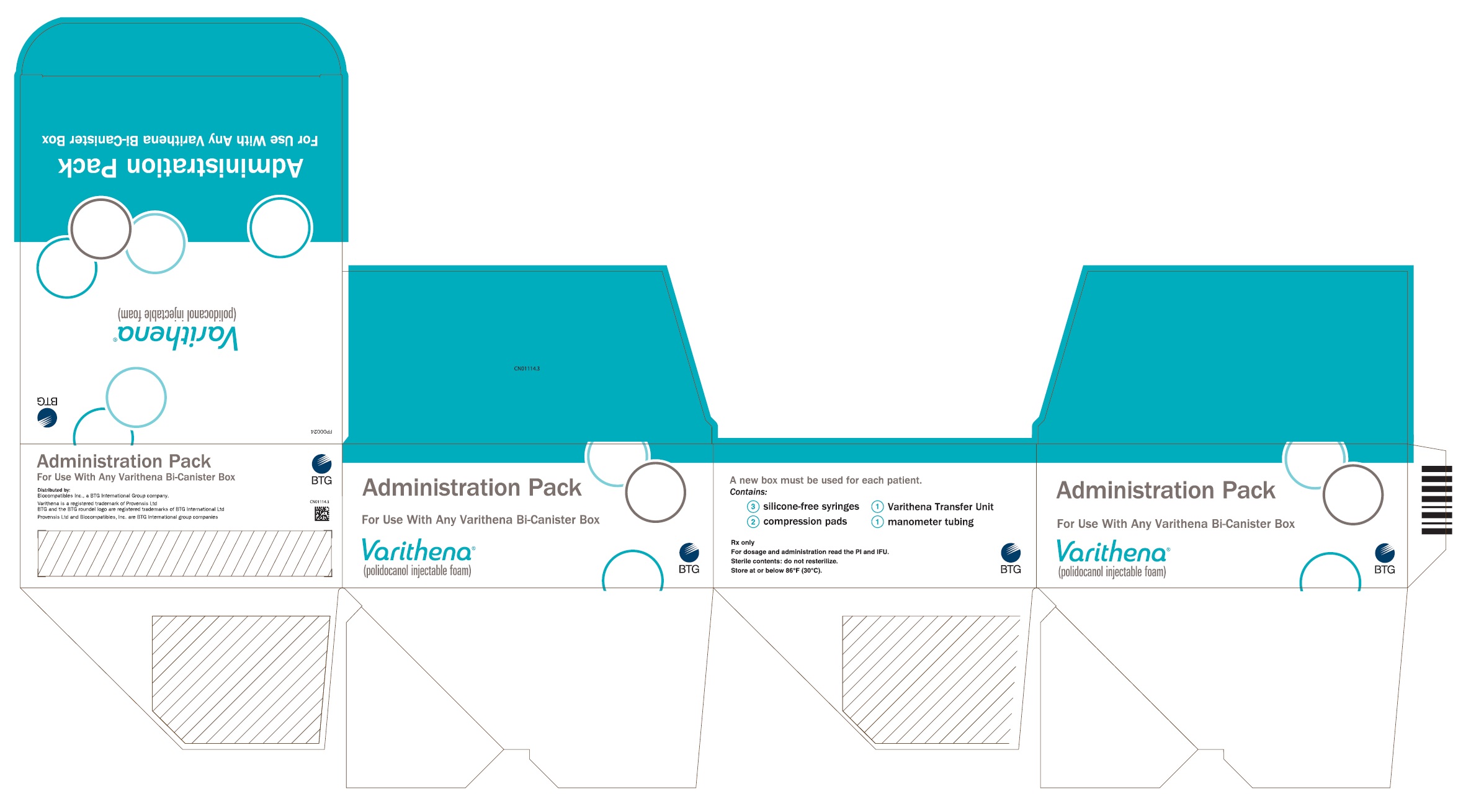
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Varithena Universal Administration Pack
-
INGREDIENTS AND APPEARANCEProduct Information