Label: VARIBAR PUDDING- barium sulfate paste
- NDC Code(s): 32909-125-22, 32909-125-54
- Packager: E-Z-EM Canada Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information - needed to use VARIBAR PUDDING safely and effectively. See full prescribing - information for VARIBAR PUDDING. VARIBAR PUDDING (barium - sulfate) oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVARIBAR PUDDING is indicated for modified barium swallow examinations - to evaluate the oral and pharyngeal function and morphology in adult - and pediatric patients 6 months of age and older.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended - Dosing - The recommended oral dose of VARIBAR PUDDING delivered by oral syringe or spoon: Adults 5 mL - Pediatric patients 1-3 mL - During a single modified barium swallow ...
-
3 DOSAGE FORMS AND STRENGTHSOral paste: barium sulfate (40% w/v) supplied - in a multiple-dose white polyethylene tube as a ready-to-use paste - for oral administration. Each tube contains either 30 mL or 230 mL - of paste.
-
4 CONTRAINDICATIONSVARIBAR PUDDING is contraindicated in patients with: - known or suspected perforation - of the gastrointestinal (GI) tract; - known obstruction - of the GI tract; - high risk of GI perforation such ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Reactions - Barium sulfate preparations contain a number of excipients, including - natural and artificial flavors and may induce serious hypersensitivity - reactions. The ...
-
6 ADVERSE REACTIONSThe following adverse reactions have been identified from - spontaneous reporting or clinical studies of barium sulfate administered - orally. Because the reactions are reported voluntarily from a ...
-
8 USE IN SPECIFIC POPULATIONS8.1 - Pregnancy - Risk Summary - VARIBAR PUDDING is not absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure to the drug. [see Clinical ...
-
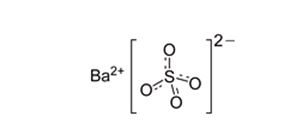
11 DESCRIPTIONVARIBAR PUDDING (barium sulfate) is a radiographic contrast agent that is supplied as a 40 % w/v ready to use paste with a vanilla aroma for oral administration. The active ingredient barium ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Due to its high atomic number, barium (the active ingredient - in VARIBAR PUDDING) is opaque to x-rays and therefore acts as a positive - contrast agent for radiographic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No animal studies have been performed to evaluate the carcinogenic potential of barium sulfate or potential effects on ...
-
16 HOW SUPPLIED/STORAGE
AND HANDLINGVARIBAR PUDDING is supplied as a paste in a multiple-dose polyethylene - tube containing either 30 mL or 230 mL of barium sulfate (40 % w/v). Provided as: 24 X 30 mL tubes - (NDC 32909-125-54); 12 X ...
-
17 PATIENT COUNSELING INFORMATIONAfter administration, advise patients to: Maintain adequate hydration. Seek medical attention for worsening of constipation or - slow gastrointestinal passage. Seek medical attention for any ...
-
PRINCIPAL DISPLAY PANEL230 mL - Varibar Pudding Label - 230 mL Varibar Pudding - Tube - 30 mL Varibar Pudding Label - 30 mL Varibar Pudding Tube
-
INGREDIENTS AND APPEARANCEProduct Information