Label: VANIQA- eflornithine hydrochloride cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5124-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 67402-040
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 18, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
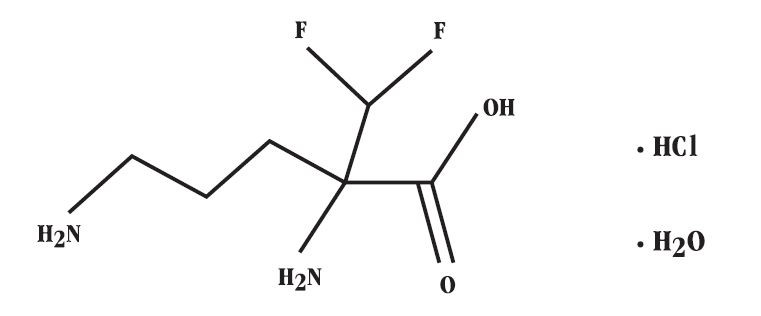
DESCRIPTIONVANIQA is a cream containing 13.9% (139 mg/g) of anhydrous eflornithine hydrochloride as eflornithine hydrochloride monohydrate (150 mg/g). Chemically, eflornithine hydrochloride is (± ...
- CLINICAL PHARMACOLOGY
-
PharmacodynamicsThere are no studies examining the inhibition of the enzyme ornithine decarboxylase (ODC) in human skin following the application of topical eflornithine. However, there are studies in the ...
-
PharmacokineticsThe mean percutaneous absorption of eflornithine in women with unwanted facial hair, from a 13.9% w/w cream formulation, is < 1% of the radioactive dose, following either single or multiple doses ...
-
INDICATIONS AND USAGEVANIQA (eflornithine hydrochloride) Cream, 13.9% is indicated for the reduction of unwanted facial hair in women. VANIQA has only been studied on the face and adjacent involved areas under the ...
-
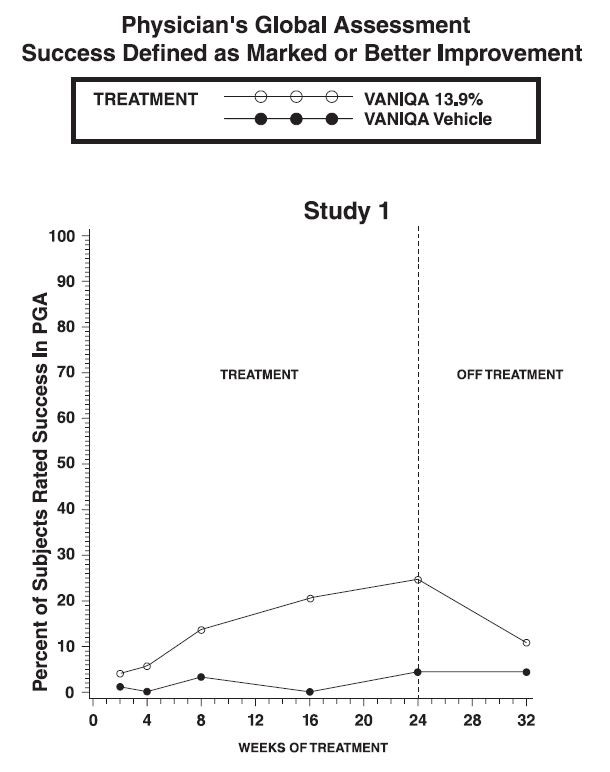
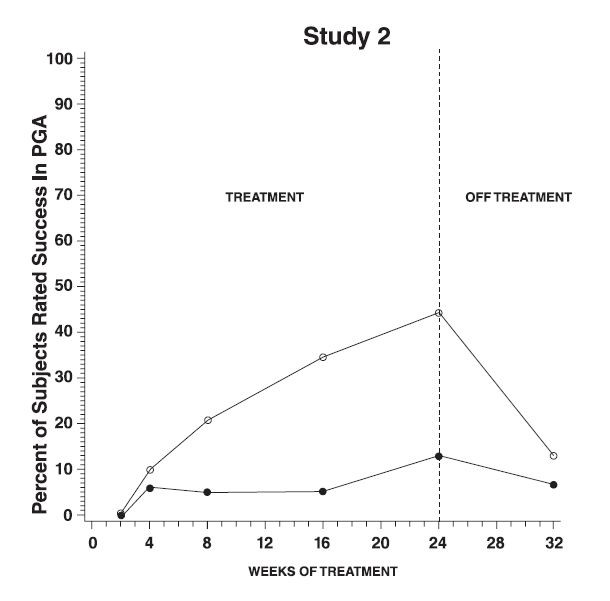
CLINICAL TRIALSResults of topical dermal studies for contact sensitization, photocontact sensitization, and photocontact irritation reveal that under conditions of clinical use, VANIQA is not expected to cause ...
-
CONTRAINDICATIONSVANIQA is contraindicated in patients with a history of sensitivity to any components of the preparation.
-
WARNINGSDiscontinue use if hypersensitivity occurs.
-
PRECAUTIONSGeneral - For external use only. Transient stinging or burning may occur when applied to abraded or broken skin.
-
INFORMATION FOR PATIENTSPatients using VANIQA should receive the following information and instructions: This medication is not a depilatory, but rather appears to retard hair growth to improve the condition and the ...
-
DRUG INTERACTIONSIt is not known if VANIQA has any interaction with other topically applied drug products. Carcinogenesis, Mutagenesis and Impairment of Fertility - In a 12-month photocarcinogenicity study in ...
-
ADVERSE REACTIONSAdverse events reported for most body systems occurred at similar frequencies in VANIQA (eflornithine hydrochloride) Cream, 13.9% and vehicle control groups, The most frequent adverse events ...
-
OVERDOSAGEOverdosage information with VANIQA is unavailable. Given the low percutaneous penetration of this drug, overdosage via the topical route is not expected (see CLINICAL PHARMACOLOGY). However ...
-
DOSAGE & ADMINISTRATIONApply a thin layer of VANIQA (eflornithine hydrochloride) Cream, 13.9% to affected areas of the face and adjacent involved areas under the chin and rub in thoroughly. Do not wash treated area for ...
-
HOW SUPPLIEDVANIQA (eflornithine hydrochloride) Cream, 13.9% is available as: 30 gram tube - NDC 54868-5124-0
-
STORAGEStore at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F) [See USP Controlled Room Temperature] Do not freeze. See tube crimp and carton end for expiration date and lot number. Rx ...
-
Patient Information Leaflet for VANIQA® (eflornithine hydrochloride) Cream, 13.9% INFORMATION FOR PATIENTS - This section contains important information about VANIQA that you should read before you begin treatment. This section ...
-
PACKAGE LABELNDC 54868-5124-0 - VANIQA®(eflornithine hydrochloride) Cream, 13.9% Rx only - SkinMedica® Net Wt 30g / 1.06 oz - Warning: Keep out of reach of children. Each gram contains: 13.9% (139 mg/g ...
-
INGREDIENTS AND APPEARANCEProduct Information