Label: VALSTAR- valrubicin solution, concentrate
- NDC Code(s): 67979-001-01
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VALSTAR safely and effectively. See full prescribing information for VALSTAR. VALSTAR - ®(valrubicin) solution, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVALSTAR is an anthracycline topoisomerase inhibitor indicated for intravesical therapy of BCG-refractory carcinoma - in situ(CIS) of the urinary bladder in patients for whom immediate cystectomy ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - For Intravesical Use Only. Do NOT administer by intravenous or intramuscular routes. VALSTAR is recommended at a dose of 800 mg administered intravesically once a ...
-
3 DOSAGE FORMS AND STRENGTHS200 mg/5 mL sterile, clear red, solution in single-use vials for intravesical instillation upon dilution.
-
4 CONTRAINDICATIONSVALSTAR is contraindicated in patients with: Perforated bladder [ see Warnings and Precautions ( 5.2)] Known hypersensitivity to anthracyclines or polyoxyl castor oil - Active ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Metastatic Bladder Cancer with Delayed Cystectomy - Inform patients that VALSTAR has been shown to induce complete response in only about 1 in 5 patients with BCG-refractory CIS, and ...
-
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNo drug interaction studies were conducted.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animal studies and its mechanism of action, VALSTAR can cause fetal harm when administered to a pregnant females - [see Clinical ...
-
10 OVERDOSAGEThere is no known antidote for overdoses of VALSTAR. The primary anticipated complications of overdosage associated with intravesical administration would be consistent with irritable bladder ...
-
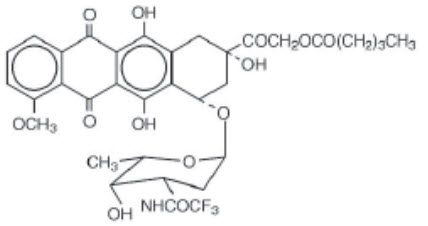
11 DESCRIPTIONVALSTAR contains valrubicin (N-trifluoroacetyladriamycin-14-valerate), which is a semisynthetic analog of the anthracycline doxorubicin as a cytotoxic agent. The chemical name of valrubicin is (2 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Valrubicin is an anthracycline that affects a variety of interrelated biological functions, most of which involve nucleic acid metabolism. In cells, it inhibits the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - The carcinogenic potential of valrubicin has not been evaluated. In vitro, valrubicin was mutagenic in the bacterial reverse mutation ...
-
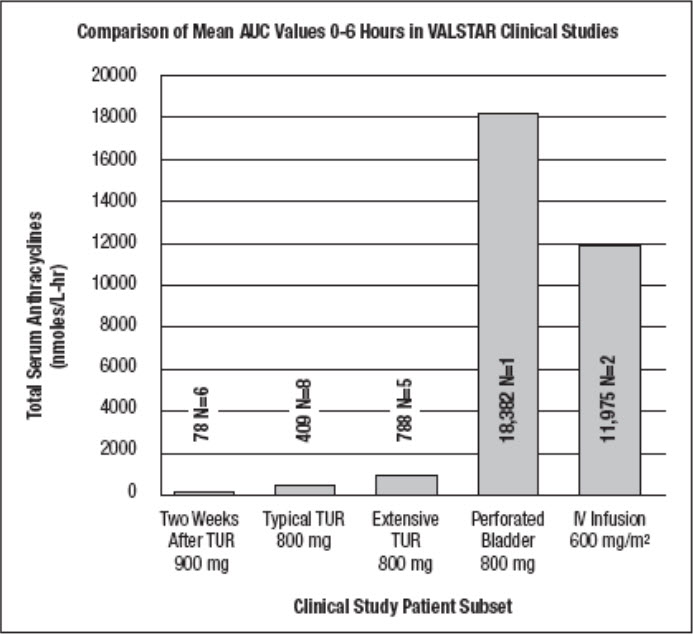
14 CLINICAL STUDIESVALSTAR was administered intravesically to a total of 230 patients with transitional cell carcinoma of the bladder, including 205 patients who received multiple weekly doses ranging from 200 to ...
-
15 REFERENCESOSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVALSTAR is a sterile, clear red solution in polyoxyl castor oil/dehydrated alcohol, USP, containing 40 mg valrubicin per mL. VALSTAR is available in single-use, clear glass vials, individually ...
-
17 PATIENT COUNSELING INFORMATIONRisk of Metastatic Bladder Cancer with Delayed Cystectomy - Inform patients that VALSTAR has been shown to induce complete responses in only about 1 in 5 patients, and that delaying cystectomy ...
-
Package Label – Principle Display Panel – Carton – 4 Vials

-
INGREDIENTS AND APPEARANCEProduct Information