Label: VALCHLOR- mechlorethamine hydrochloride gel
- NDC Code(s): 69639-120-01
- Packager: Helsinn Therapeutics (U.S.), Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VALCHLOR - ® safely and effectively. See full prescribing information for VALCHLOR. VALCHLOR (mechlorethamine) gel ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVALCHLOR is indicated for the topical treatment of Stage IA and IB mycosis fungoides-type cutaneous T-cell lymphoma in patients who have received prior skin-directed therapy.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing and Dose Modification - For Topical Dermatological Use Only - Apply a thin film of VALCHLOR gel once daily to affected areas of the skin. Stop treatment with VALCHLOR for any grade ...
-
3 DOSAGE FORMS AND STRENGTHSThe active ingredient in VALCHLOR is mechlorethamine. Each tube of VALCHLOR contains 60g of 0.016% w/w mechlorethamine clear gel (equivalent to 0.02% mechlorethamine HCl).
-
4 CONTRAINDICATIONSThe use of VALCHLOR is contraindicated in patients with known severe hypersensitivity to mechlorethamine. Hypersensitivity reactions, including anaphylaxis, have occurred with topical formulations ...
-
5 WARNINGS AND PRECAUTIONS5.1 Mucosal or Eye Injury - Exposure of the eyes to mechlorethamine causes pain, burns, inflammation, photophobia, and blurred vision. Blindness and severe irreversible anterior eye injury may ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the prescribing information: Mucosal or eye injury [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSNo drug interaction studies have been performed with VALCHLOR. Systemic exposure has not been observed with topical administration of VALCHLOR; therefore, systemic drug interactions are not ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on case reports in humans, findings in animal reproduction studies, its mechanism of action, and genotoxicity findings, mechlorethamine may cause fetal ...
-
11 DESCRIPTIONVALCHLOR is a topical product that contains mechlorethamine HCl, an alkylating drug. Mechlorethamine HCl is a white to off white solid that is very soluble in water and methanol, partially ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mechlorethamine, also known as nitrogen mustard, is an alkylating agent which inhibits rapidly proliferating cells. 12.3 Pharmacokinetics - Systemic exposure was ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Mechlorethamine was carcinogenic in mice when injected intravenously with four doses of 2.4 mg/kg (0.1% solution) at 2-week intervals ...
-
14 CLINICAL STUDIESThe efficacy of VALCHLOR was assessed in a randomized, multicenter, observer-blind, active-controlled, non-inferiority clinical trial of 260 patients with Stage IA, IB, and IIA mycosis ...
-
15 REFERENCES1 OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVALCHLOR is supplied in 60g tubes of 0.016% w/w mechlorethamine as a clear gel [NDC 69639-120-01]. Prior to dispensing, store in the freezer at -13°F to 5°F (-25°C to -15°C). Advise patients ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Medication Guide) Advise patients of the following and provide a copy of the Medication Guide. Instructions for Patients and Caregivers for ...
-
MEDICATION GUIDEMEDICATION GUIDE - VALCHLOR ® (val-klor) (mechlorethamine) gel - Important information: VALCHLOR is for use on skin only. Do not get VALCHLOR near or in your eyes, mouth, or nose. What is ...
-
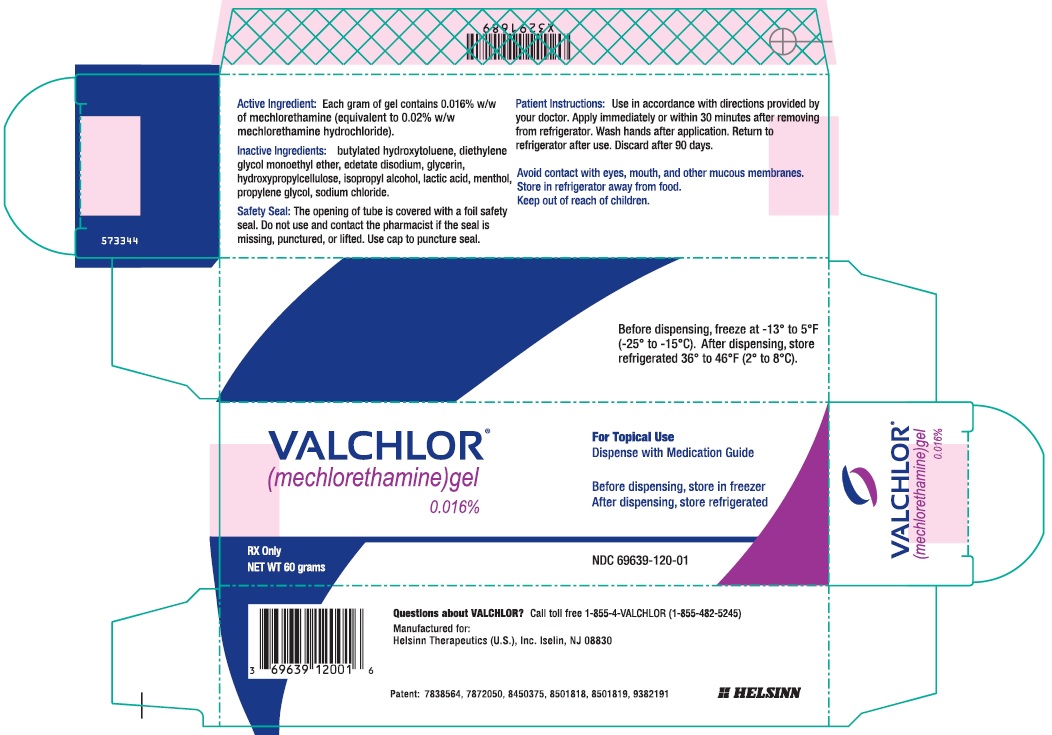
Principal Display Panel - 60 g Tube CartonVALCHLOR® (mechlorethamine) gel - 0.016% For Topical Use - Dispense with Medication Guide - Before dispensing, store in freezer ...
-
Tube Label - Front

-
Tube Label - Back

-
INGREDIENTS AND APPEARANCEProduct Information