Label: UPTRAVI- selexipag tablet, coated
UPTRAVI TITRATION PACK- selexipag kit
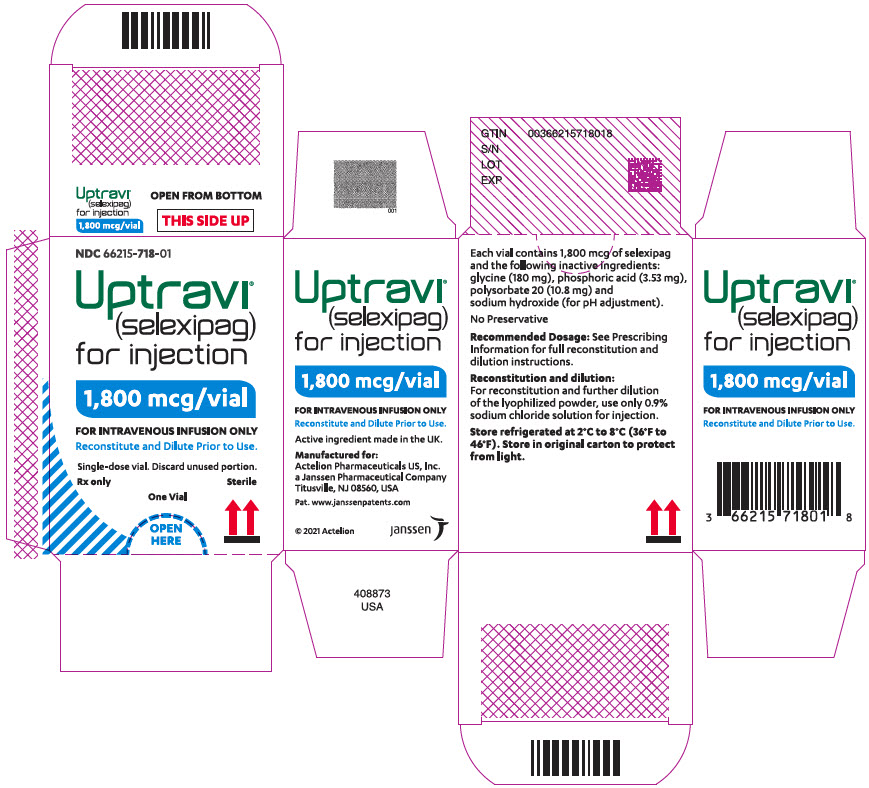
UPTRAVI- selexipag injection, powder, for solution
- NDC Code(s): 66215-602-06, 66215-602-14, 66215-604-06, 66215-606-06, view more
- Packager: Actelion Pharmaceuticals US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use UPTRAVI safely and effectively. See full prescribing information for UPTRAVI. UPTRAVI - ® (selexipag) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Pulmonary Arterial Hypertension - UPTRAVI is indicated for the treatment of pulmonary arterial hypertension (PAH, WHO Group I) to delay disease progression and reduce the risk of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - UPTRAVI Film-coated Tablets - The recommended starting dosage of UPTRAVI tablets is 200 micrograms (mcg) given twice daily. Tolerability may be improved when taken ...
-
3 DOSAGE FORMS AND STRENGTHSUPTRAVI is available in the following presentations: Film-Coated Tablets - 200 mcg selexipag [Light yellow tablet debossed with 2] 400 mcg selexipag [Red tablet debossed with 4] 600 mcg selexipag ...
-
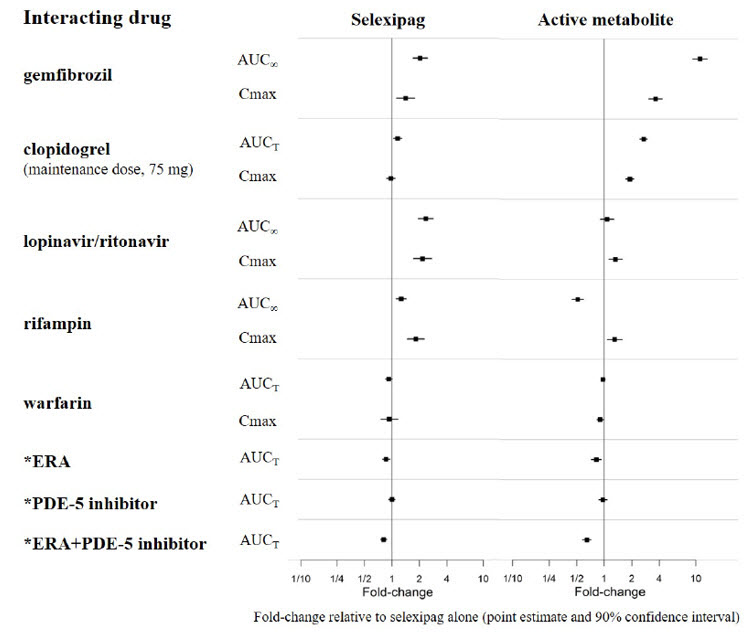
4 CONTRAINDICATIONSHypersensitivity to the active substance or to any of the excipients. Concomitant use of strong inhibitors of CYP2C8 (e.g., gemfibrozil) [see - Drug Interactions (7.1 ...
-
5 WARNINGS AND PRECAUTIONS5.1 Pulmonary Edema with Pulmonary Veno-Occlusive Disease - Should signs of pulmonary edema occur, consider the possibility of associated pulmonary veno-occlusive disease. If confirmed ...
-
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared ...
-
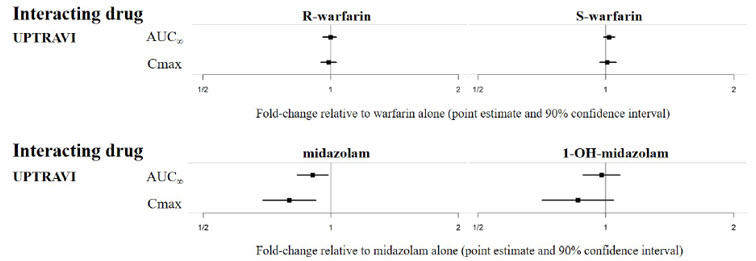
7 DRUG INTERACTIONS7.1 CYP2C8 Inhibitors - Concomitant administration with gemfibrozil, a strong inhibitor of CYP2C8, doubled the exposure to selexipag and increased exposure to the active metabolite by ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies with UPTRAVI in pregnant women. Animal reproduction studies performed with selexipag showed no clinically ...
-
10 OVERDOSAGEIsolated cases of overdose with UPTRAVI tablets up to 3200 mcg were reported. Mild, transient nausea was the only reported consequence. In the event of overdose, supportive measures must be taken ...
-
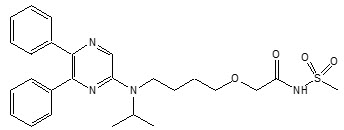
11 DESCRIPTIONUPTRAVI contains selexipag, a prostacyclin receptor agonist. The chemical name of selexipag is 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}- N-(methylsulfonyl) acetamide ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Selexipag is a prostacyclin receptor (IP receptor) agonist that is structurally distinct from prostacyclin. Selexipag is hydrolyzed by carboxylesterase 1 to yield its ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: In the 2-year carcinogenicity studies, chronic oral administration of selexipag revealed no evidence of ...
-
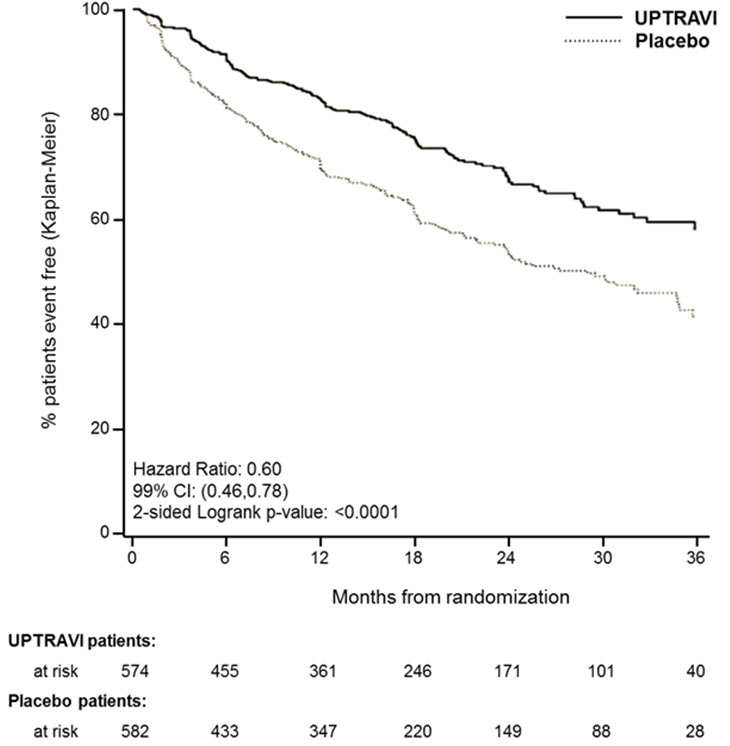
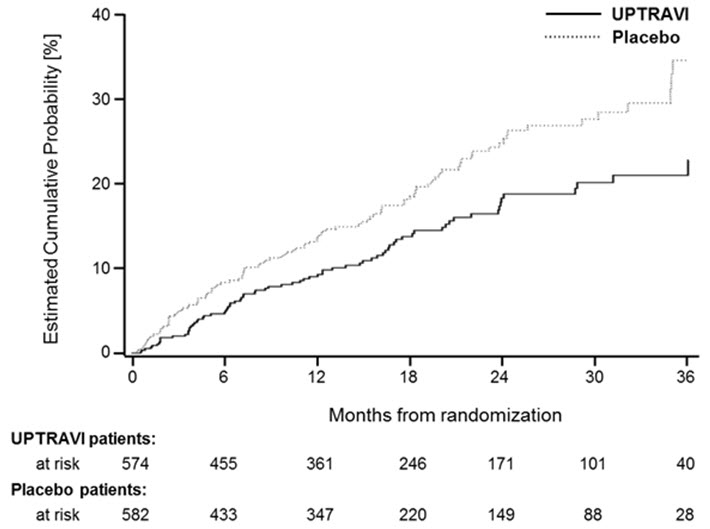
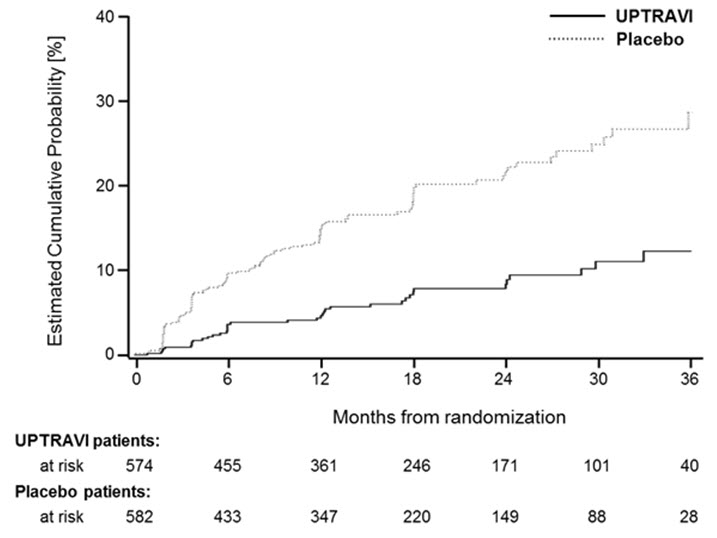
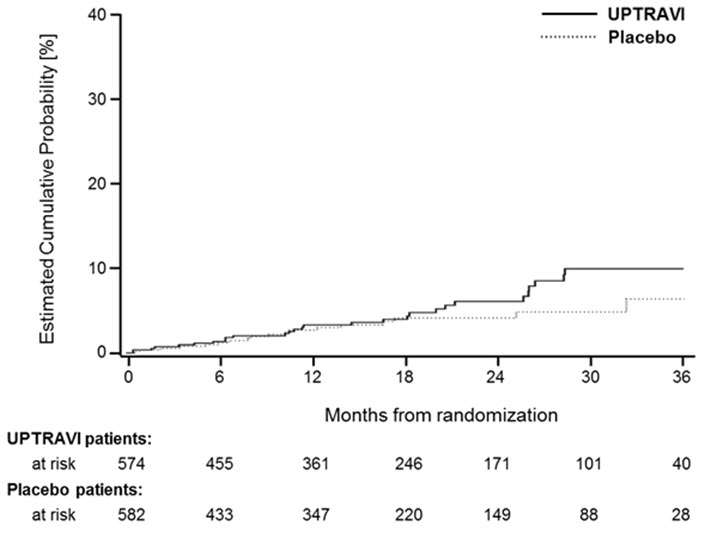
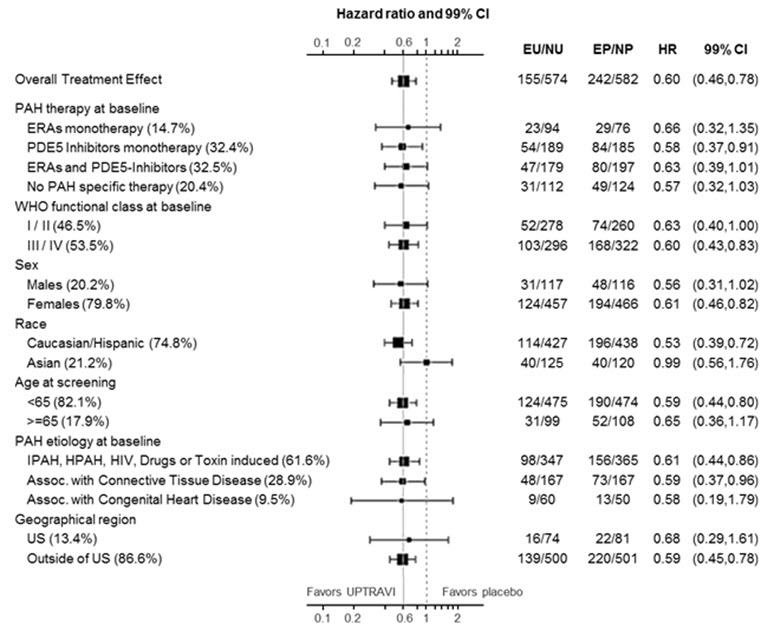
14 CLINICAL STUDIES14.1 Efficacy of UPTRAVI Tablets in Patients with Pulmonary Arterial Hypertension - The effect of UPTRAVI tablets on progression of PAH was demonstrated in a multi-center, double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGUPTRAVI - ® (selexipag) film-coated, round tablets are supplied in the following configurations: Strength - (mcg ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Inform Patients: To take a missed dose as soon as possible, unless the next dose is within the next 6 ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Actelion Pharmaceuticals US, Inc. a Janssen Pharmaceutical Company - Titusville, NJ 08560, USA - JN20220728 - For patent information ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 07/2022 - PATIENT INFORMATION - UPTRAVI - ® (up-TRA-vee ...
-
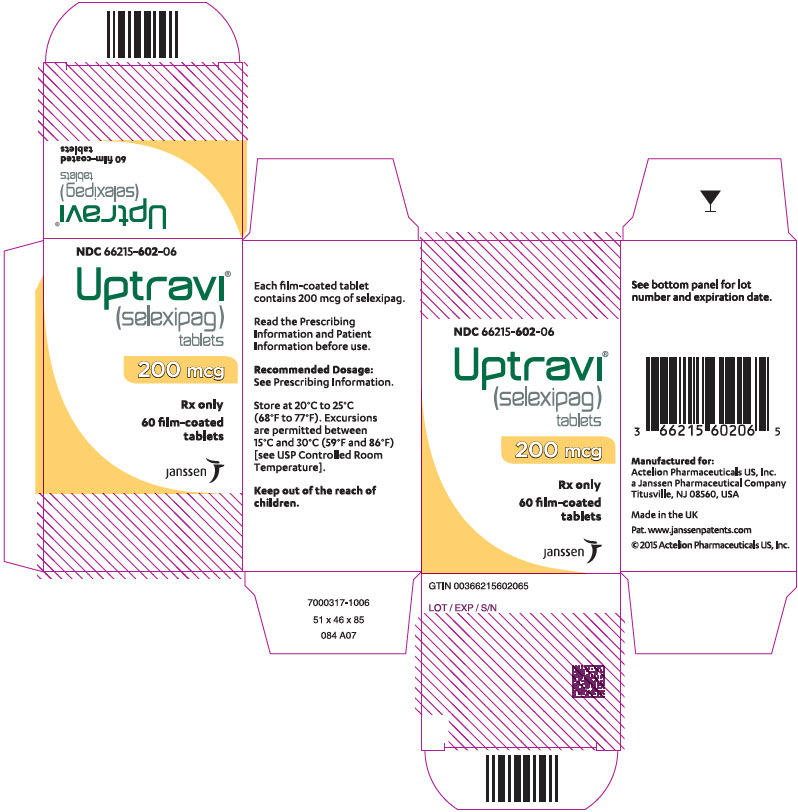
PRINCIPAL DISPLAY PANEL - 200 mcg Tablet Bottle CartonNDC 66215-602-06 - Uptravi - ® (selexipag) tablets - 200 mcg - Rx only - 60 film-coated - tablets - janssen
-
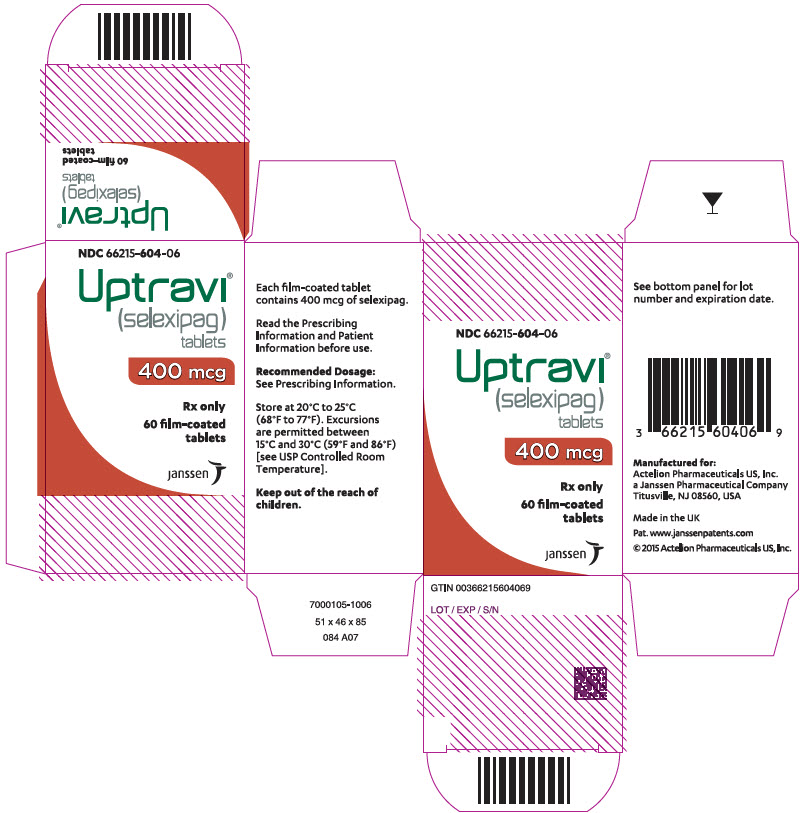
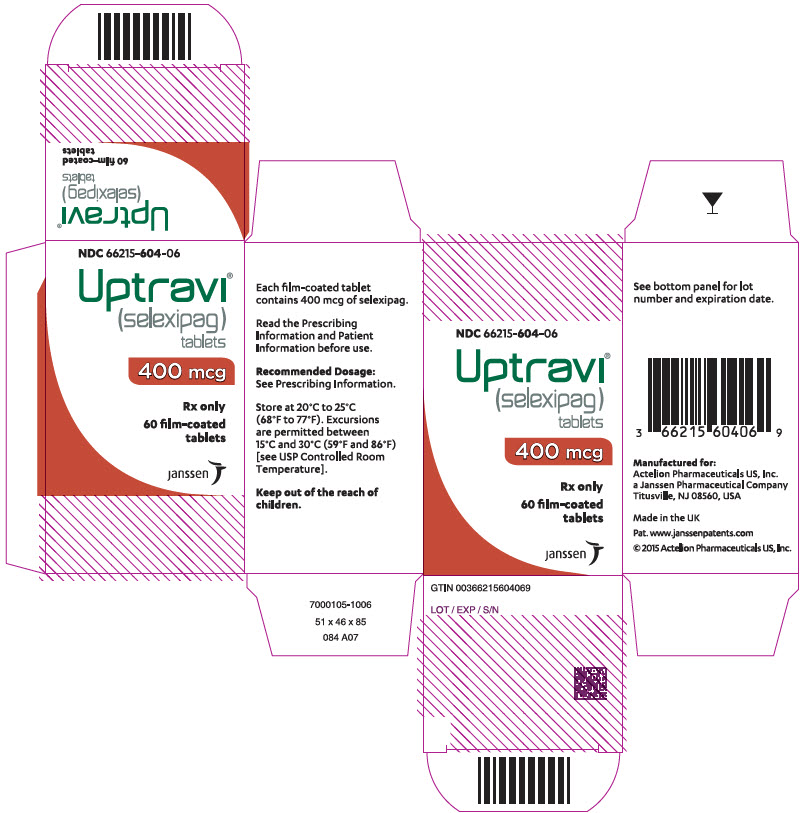
PRINCIPAL DISPLAY PANEL - 400 mcg Tablet Bottle CartonNDC 66215-604-06 - Uptravi - ® (selexipag) tablets - 400 mcg - Rx only - 60 film-coated - tablets - janssen
-
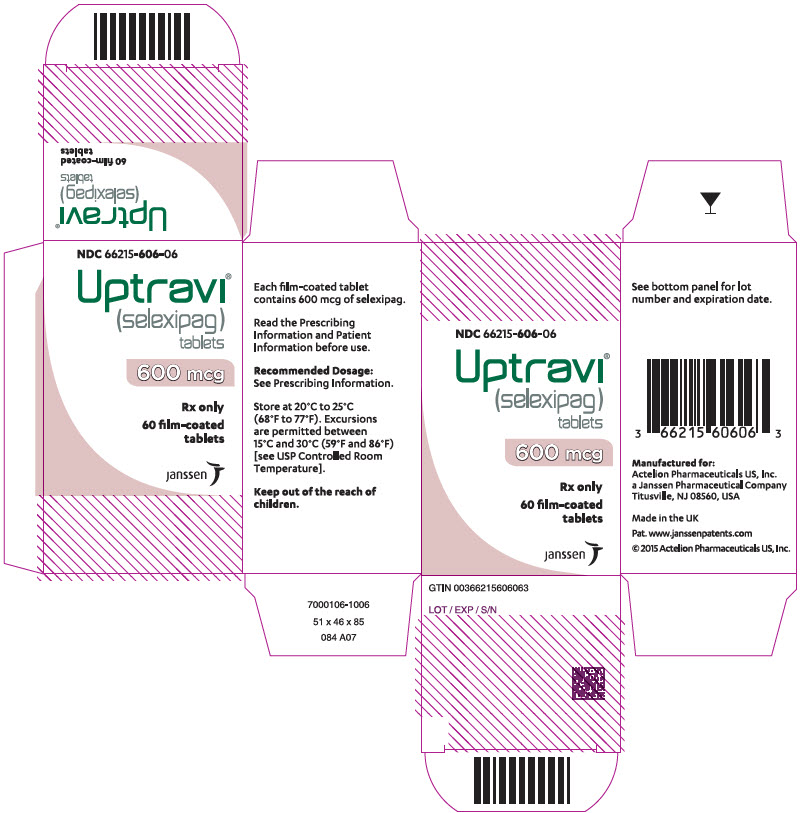
PRINCIPAL DISPLAY PANEL - 600 mcg Tablet Bottle CartonNDC 66215-606-06 - Uptravi - ® (selexipag) tablets - 600 mcg - Rx only - 60 film-coated - tablets - janssen
-
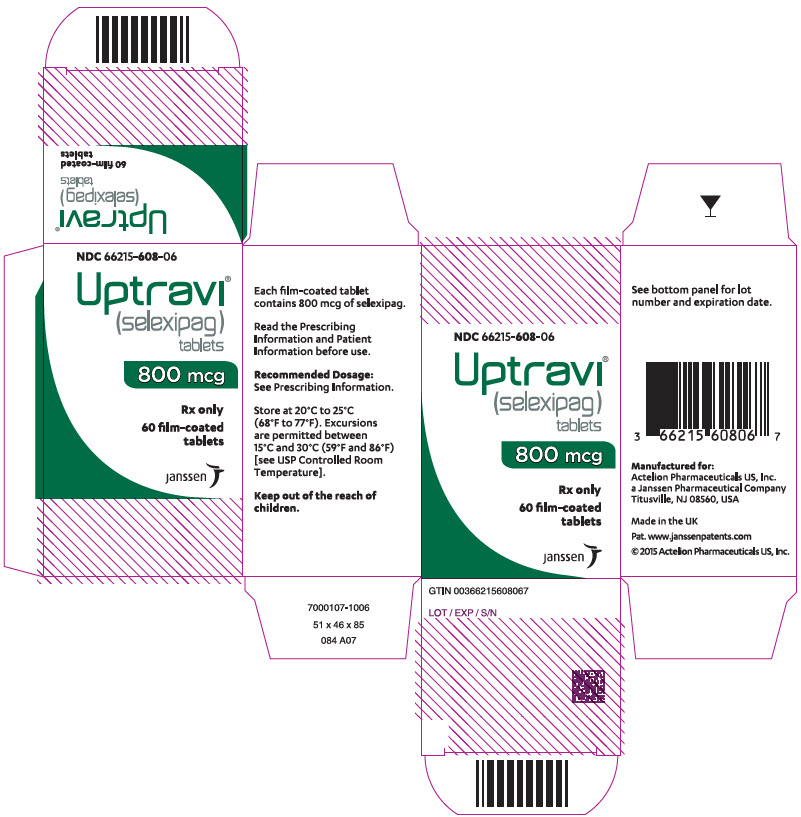
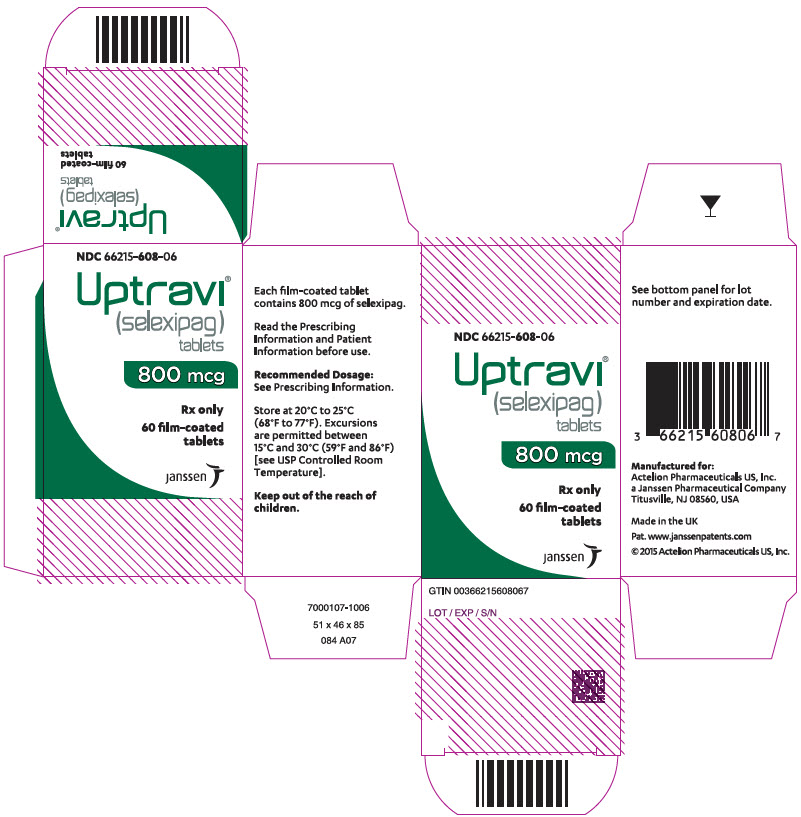
PRINCIPAL DISPLAY PANEL - 800 mcg Tablet Bottle CartonNDC 66215-608-06 - Uptravi - ® (selexipag) tablets - 800 mcg - Rx only - 60 film-coated - tablets - janssen
-
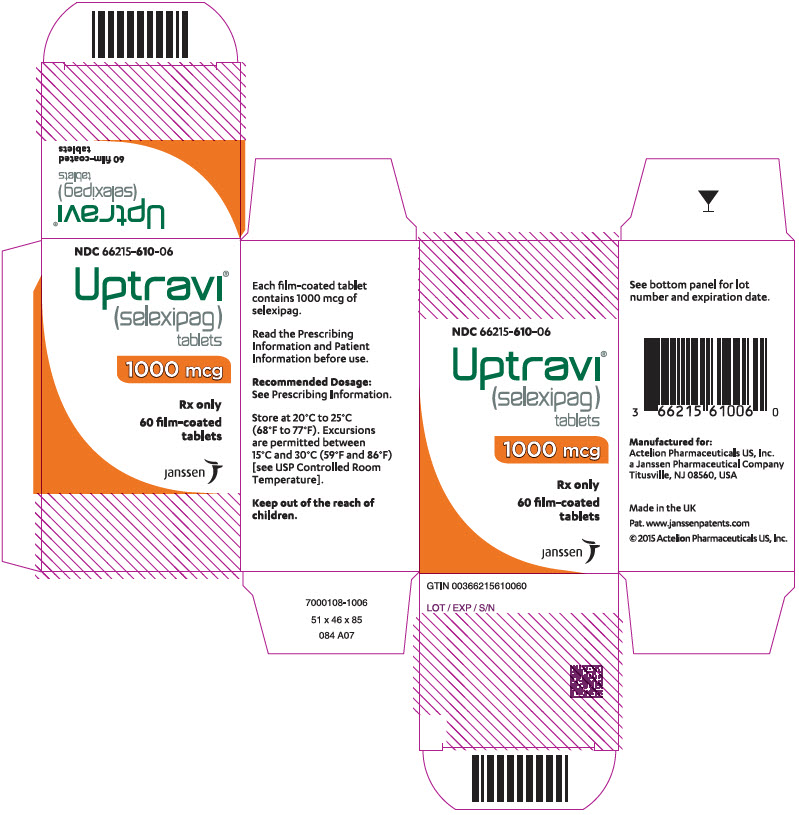
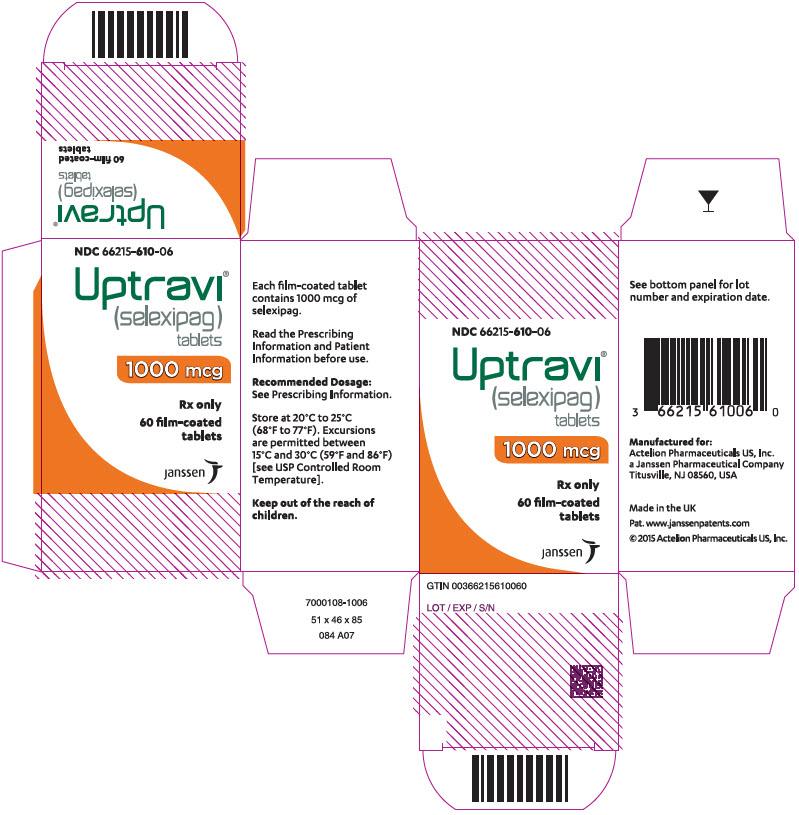
PRINCIPAL DISPLAY PANEL - 1000 mcg Tablet Bottle CartonNDC 66215-610-06 - Uptravi - ® (selexipag) tablets - 1000 mcg - Rx only - 60 film-coated - tablets - janssen
-
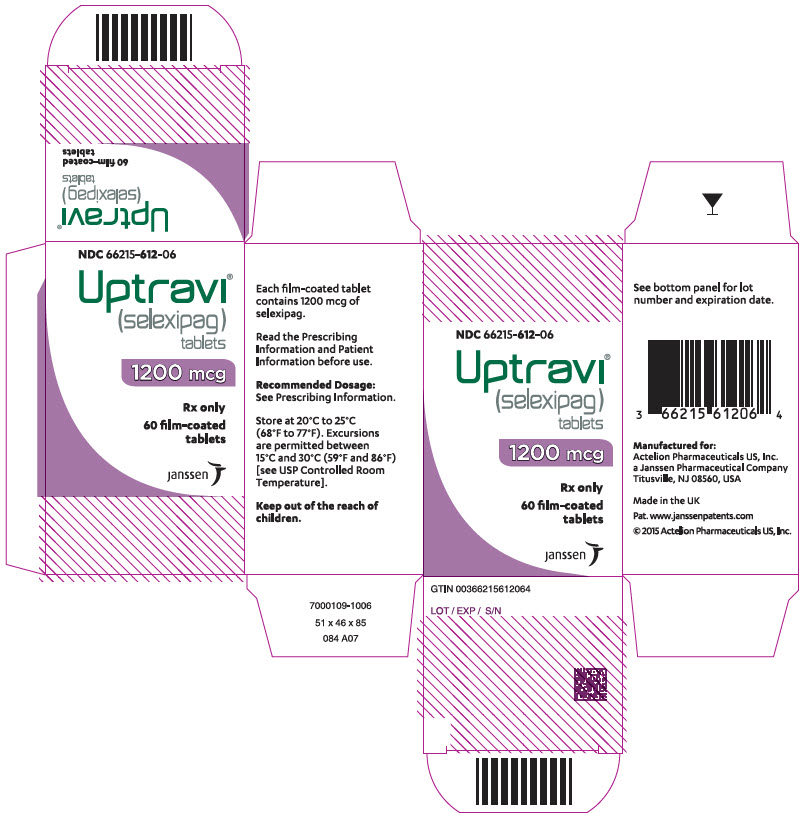
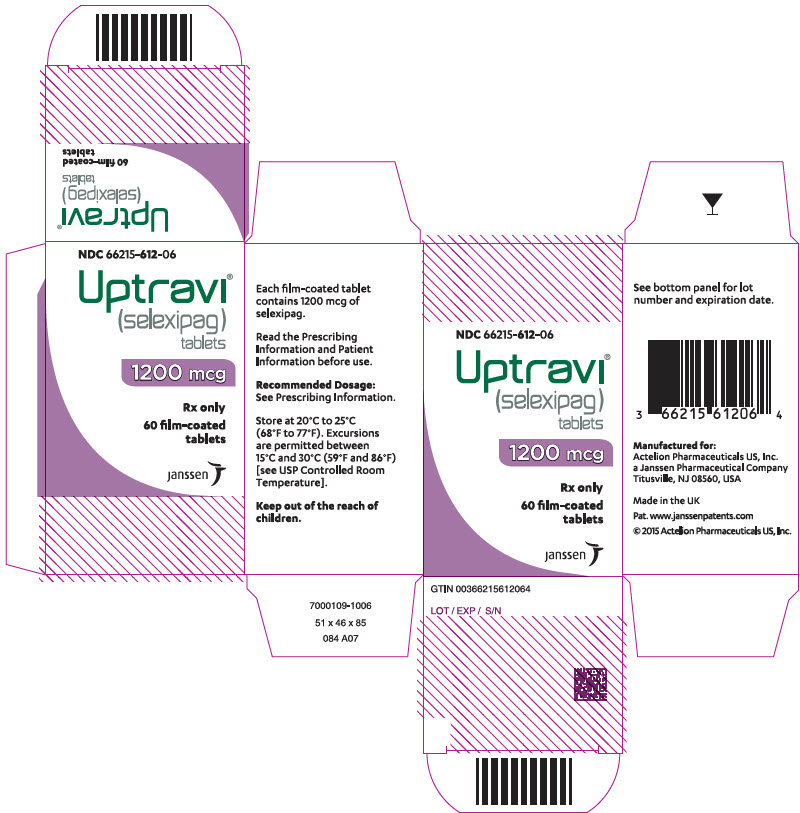
PRINCIPAL DISPLAY PANEL - 1200 mcg Tablet Bottle CartonNDC 66215-612-06 - Uptravi - ® (selexipag) tablets - 1200 mcg - Rx only - 60 film-coated - tablets - janssen
-
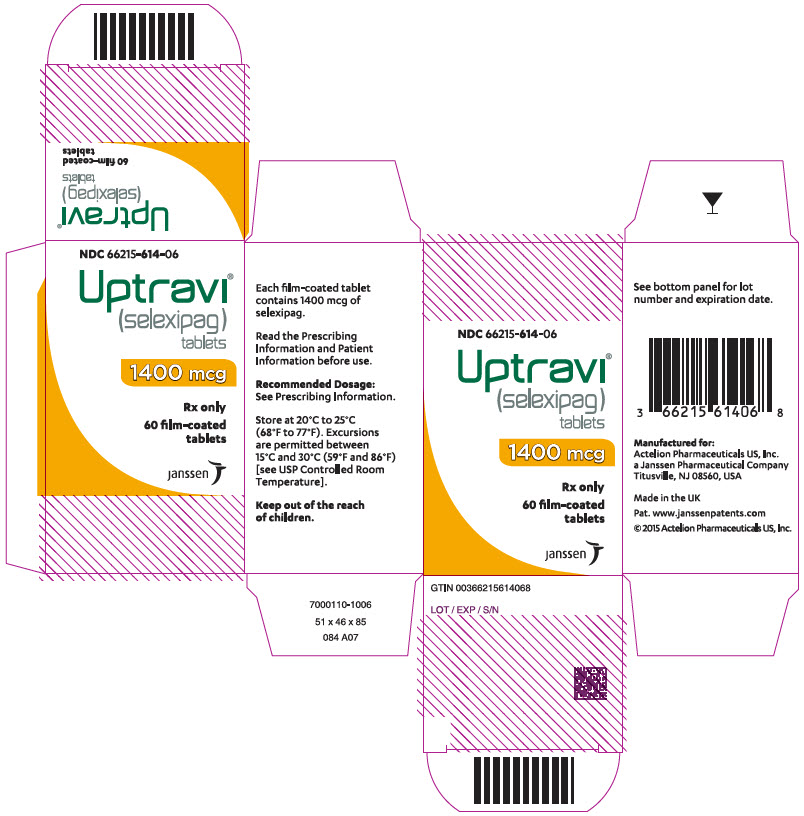
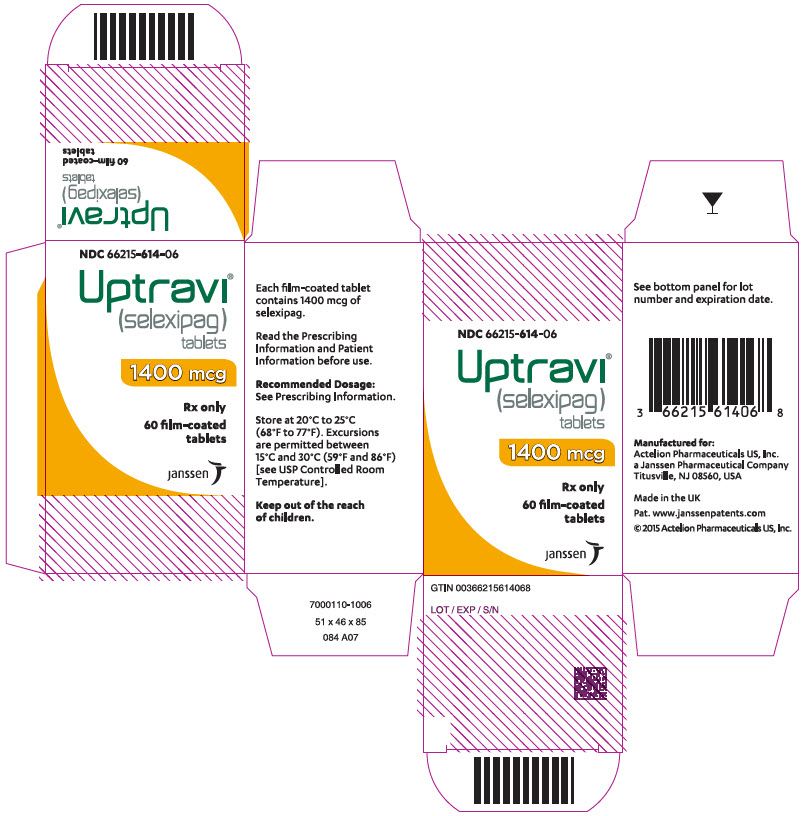
PRINCIPAL DISPLAY PANEL - 1400 mcg Tablet Bottle CartonNDC 66215-614-06 - Uptravi - ® (selexipag) tablets - 1400 mcg - Rx only - 60 film-coated - tablets - janssen
-
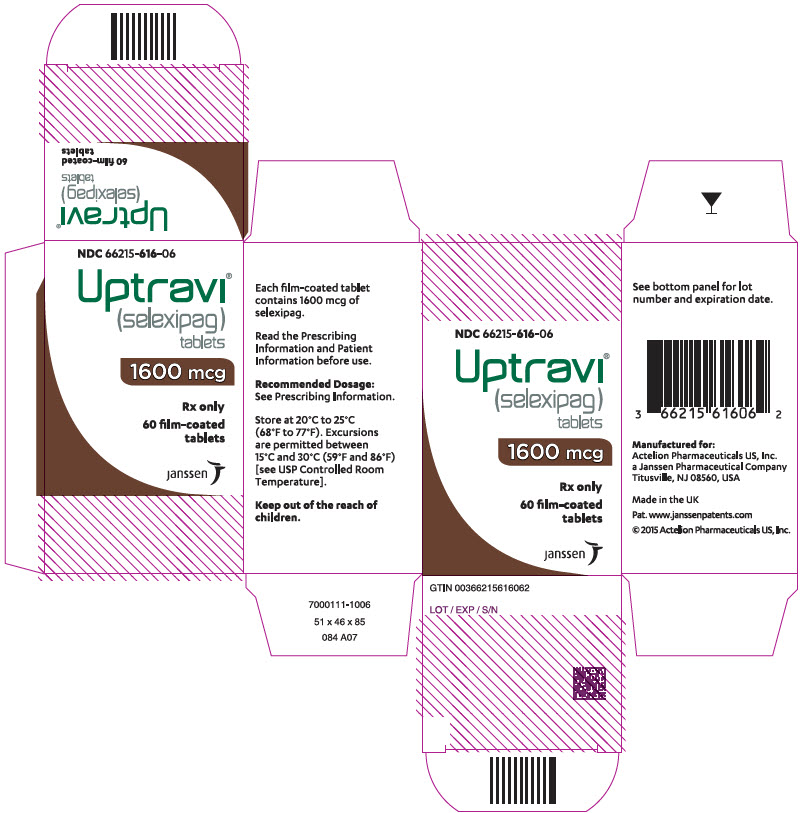
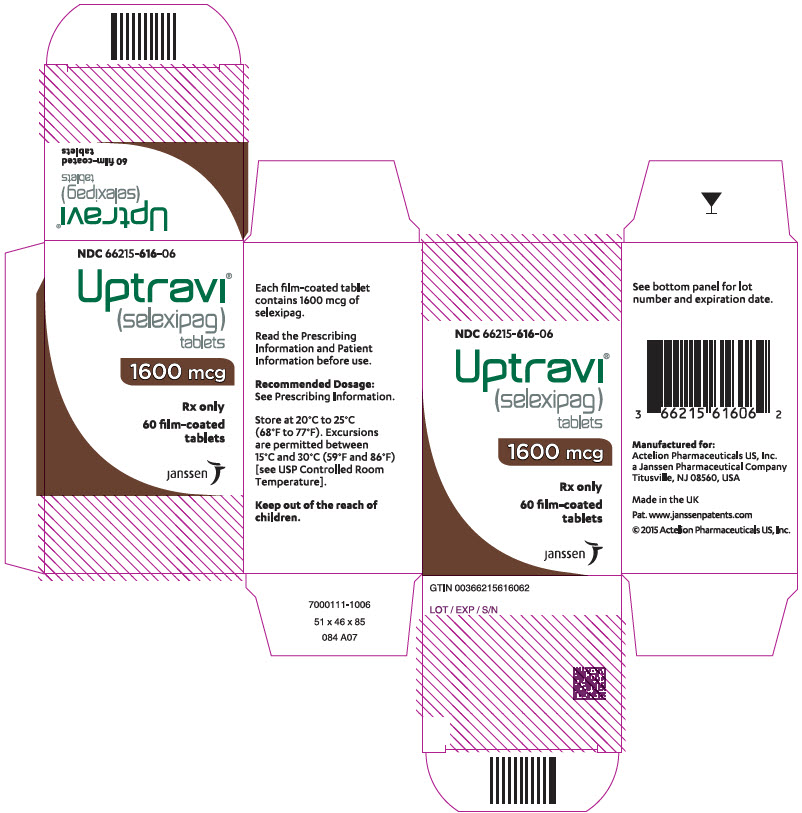
PRINCIPAL DISPLAY PANEL - 1600 mcg Tablet Bottle CartonNDC 66215-616-06 - Uptravi - ® (selexipag) tablets - 1600 mcg - Rx only - 60 film-coated - tablets - janssen
-
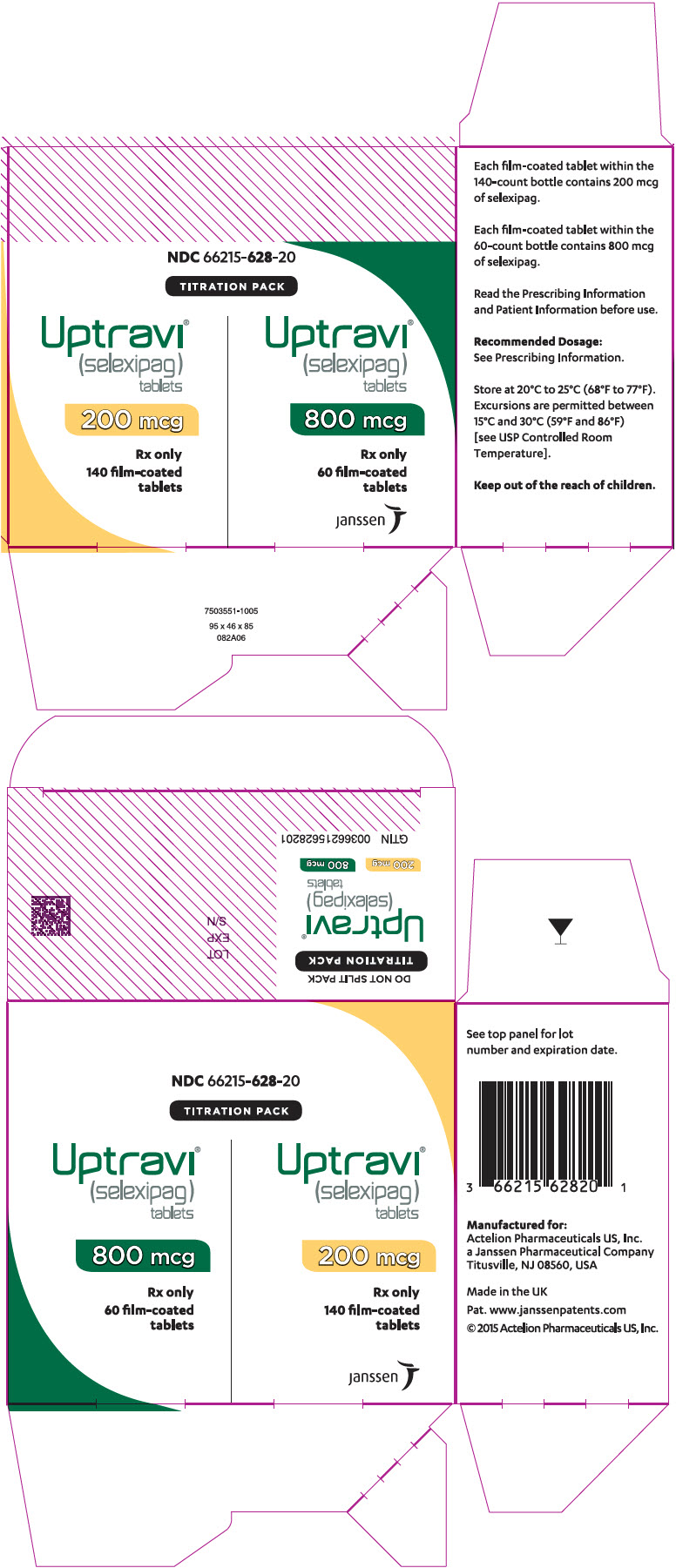
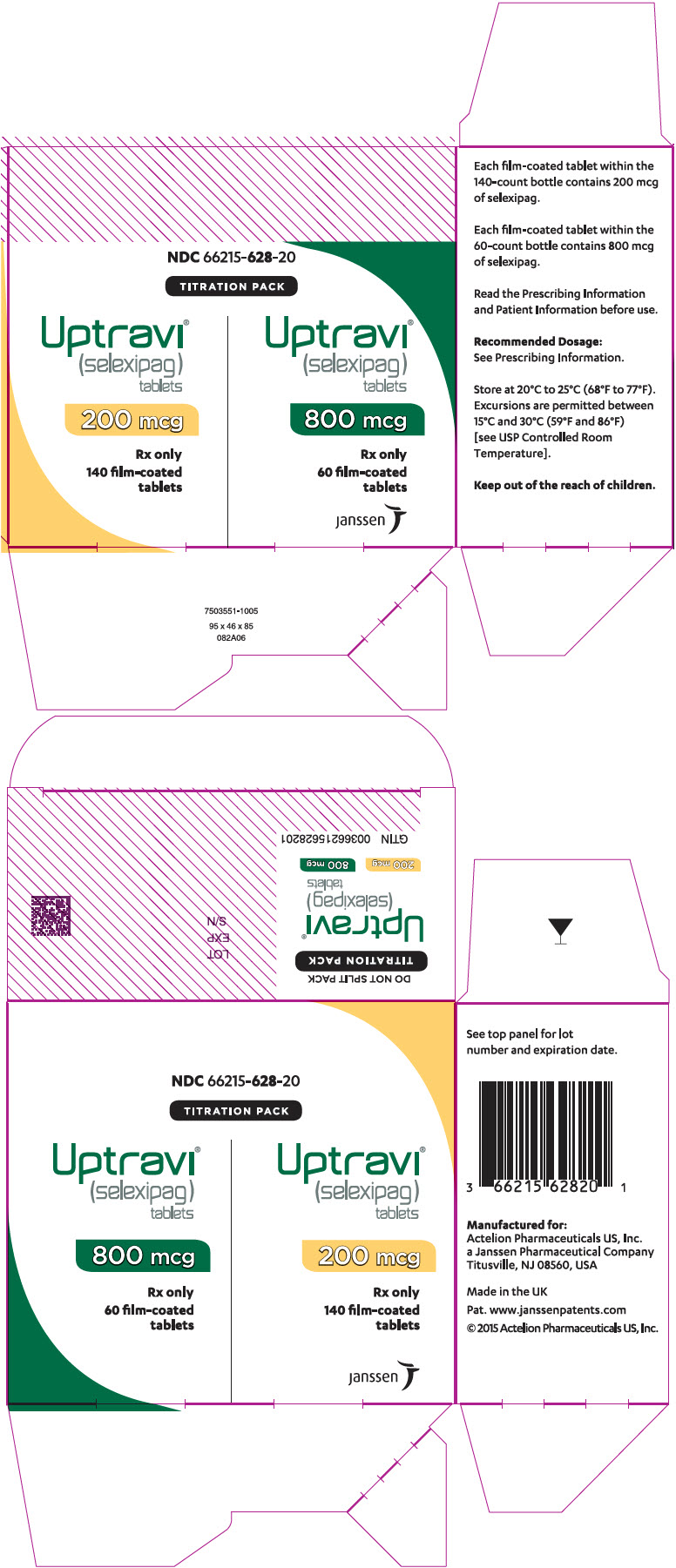
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 66215-628-20 - TITRATION PACK - Uptravi - ® (selexipag) tablets - 200 mcg - Rx only - 140 film-coated - tablets - Uptravi - ® (selexipag ...
-
PRINCIPAL DISPLAY PANEL - 1,800 mcg Vial CartonNDC 66215-718-01 - Uptravi - ® (selexipag) for injection - 1,800 mcg/vial - FOR INTRAVENOUS INFUSION ONLY - Reconstitute and Dilute Prior to Use ...
-
INGREDIENTS AND APPEARANCEProduct Information