Label: UBRELVY- ubrogepant tablet
- NDC Code(s): 0023-6498-01, 0023-6498-02, 0023-6498-04, 0023-6498-10, view more
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use UBRELVY safely and effectively. See full prescribing information for UBRELVY. UBRELVY® (ubrogepant) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
UBRELVY is indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use - UBRELVY is not indicated for the preventive treatment of migraine.
-
2
DOSAGE AND ADMINISTRATION
2.1 - Recommended Dosage - The recommended dose of UBRELVY is 50 mg or 100 mg taken orally with or without food. If needed, a second dose may be taken at least 2 hours after the initial ...
-
3
DOSAGE FORMS AND STRENGTHS
UBRELVY 50 mg is supplied as white to off-white, capsule-shaped, biconvex tablets debossed with “U50” on one side. UBRELVY 100 mg is supplied as white to off-white, capsule-shaped, biconvex ...
-
4
CONTRAINDICATIONS
UBRELVY is contraindicated: • With concomitant use of strong CYP3A4 inhibitors [see Drug Interactions (7.1)] • In patients with a history of serious hypersensitivity to ubrogepant or any ...
-
5
WARNINGS AND PRECAUTIONS
5.1 - Hypersensitivity Reactions - Hypersensitivity reactions, including anaphylaxis, dyspnea, facial or throat edema, rash, urticaria, and pruritus, have been reported with use of ...
-
6
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] 6.1 - Clinical Trials ...
-
7
DRUG INTERACTIONS
7.1 - CYP3A4 Inhibitors - Co-administration of UBRELVY with ketoconazole, a strong CYP3A4 inhibitor, resulted in a significant increase in exposure of ubrogepant [see Clinical ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors outcomes in women who become pregnant while taking UBRELVY. Patients should be ...
-
10
OVERDOSAGE
The elimination half-life of ubrogepant is approximately 5 to 7 hours; therefore, monitoring of patients after overdose with UBRELVY should continue for at least 24 hours, or while symptoms or ...
-
11
DESCRIPTION
The active ingredient of UBRELVY is ubrogepant, a calcitonin gene-related peptide (CGRP) receptor antagonist. The chemical name of ubrogepant is ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Ubrogepant is a calcitonin gene-related peptide receptor antagonist. 12.2 - Pharmacodynamics - Cardiac Electrophysiology - At a dose 2 times the ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - Two-year oral carcinogenicity studies of ubrogepant were conducted in mice (0, 5, 15, or 50 mg/kg/day) and ...
-
14
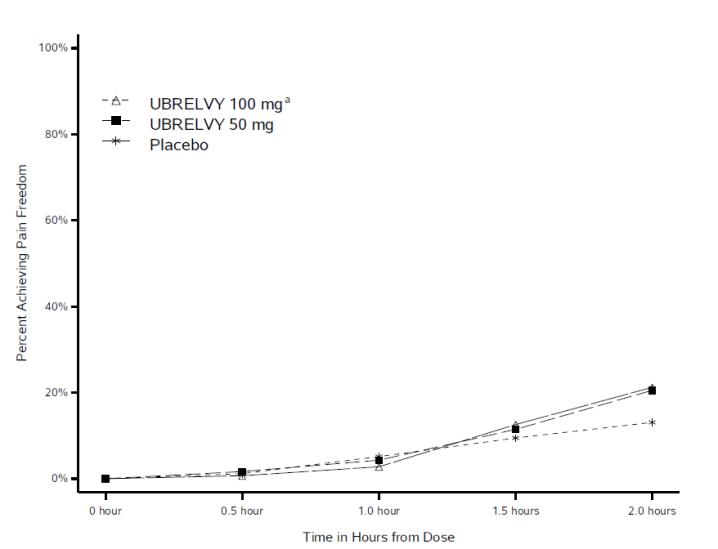
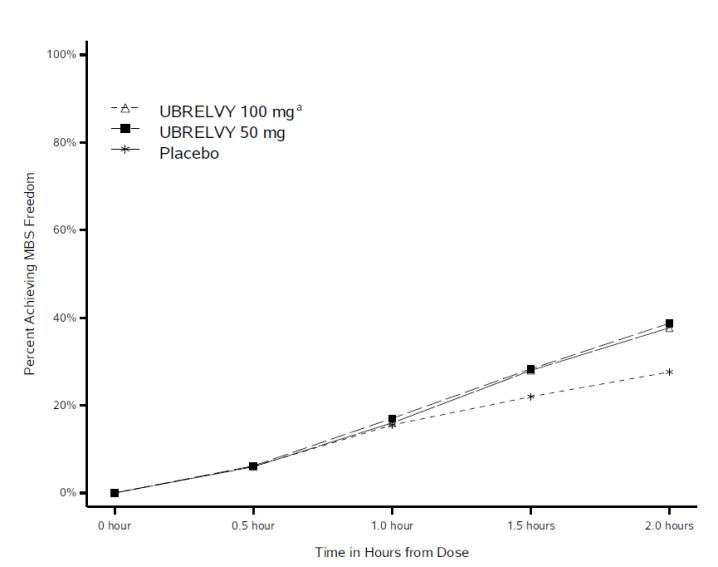
CLINICAL STUDIES
The efficacy of UBRELVY for the acute treatment of migraine was demonstrated in two randomized, double-blind, placebo-controlled trials [Study 1 (NCT02828020) and Study 2 (NCT02867709)]. Study 1 ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
16.1 - How Supplied - UBRELVY 50 mg is supplied as white to off-white, capsule-shaped, biconvex tablets debossed with “U50” on one side in unit-dose packets (each packet contains 1 ...
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Hypersensitivity Reactions - Inform patients about the signs and symptoms of hypersensitivity reactions and that ...
-
PATIENT PACKAGE INSERTPatient Information - UBRELVY® (you-brel-vee) (ubrogepant) tablets, for oral use - What is UBRELVY? UBRELVY is a prescription medicine used for the acute treatment of migraine attacks ...
-
PRINCIPAL DISPLAY PANEL
NDC 0023-6498-10 - contains - 10 tablets - Rx Only - UBRELVY® (ubrogepant) tablets - 50 mg
-
PRINCIPAL DISPLAY PANEL
NDC 0023-6501-10 - contains - 10 tablets - Rx Only - UBRELVY® (ubrogepant) tablets - 100 mg
-
INGREDIENTS AND APPEARANCEProduct Information



![The following structural formula for UBRELVY is ubrogepant, a calcitonin gene-related peptide (CGRP) receptor antagonist. The chemical name of ubrogepant is (3'S)-N-((3S,5S,6R)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide.](/dailymed/image.cfm?name=ubrelvy-01.jpg&setid=fd9f9458-fd96-4688-be3f-f77b3d1af6ab)