Label: TYMLOS- abaloparatide injection, solution
- NDC Code(s): 70539-001-01, 70539-001-02, 70539-001-98, 70539-001-99
- Packager: Radius Health, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TYMLOS safely and effectively. See full prescribing information for TYMLOS. TYMLOS® (abaloparatide) injection, for subcutaneous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Treatment of Postmenopausal Women with Osteoporosis at High Risk for Fracture - TYMLOS is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of TYMLOS is 80 mcg administered subcutaneously once daily. Patients should receive supplemental calcium and vitamin D if dietary intake is ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 3120 mcg/1.56 mL (2000 mcg/mL) of abaloparatide in clear, colorless solution in a single-patient-use prefilled pen. The prefilled pen delivers 30 doses of TYMLOS, each containing 80 mcg ...
-
4 CONTRAINDICATIONSTYMLOS is contraindicated in patients with a history of systemic hypersensitivity to abaloparatide or to any component of the product formulation. Reactions have included anaphylaxis, dyspnea, and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Osteosarcoma - Abaloparatide caused a dose-dependent increase in the incidence of osteosarcoma in male and female rats after subcutaneous administration at exposures 4 to 28 times the ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in greater detail in other sections: Orthostatic Hypotension [see Warnings and Precautions (5.2)] Hypercalcemia [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSNo specific drug-drug interaction studies have been performed [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - TYMLOS is not indicated for use in females of reproductive potential. There are no human data with TYMLOS use in pregnant women to inform any drug associated ...
-
10 OVERDOSAGEIn a clinical study, accidental overdose was reported in a patient who received 400 mcg in one day (5 times the recommended clinical dose); dosing was temporarily interrupted. The patient ...
-
11 DESCRIPTIONTYMLOS injection for subcutaneous administration contains abaloparatide, a synthetic 34 amino acid peptide. Abaloparatide is an analog of human parathyroid hormone related peptide, PTHrP(1-34). It ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Abaloparatide is a PTHrP(1-34) analog which acts as an agonist at the PTH1 receptor (PTH1R). This results in activation of the cAMP signaling pathway in target cells ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 2-year carcinogenicity study, abaloparatide was administered once daily to male and female Fischer rats by ...
-
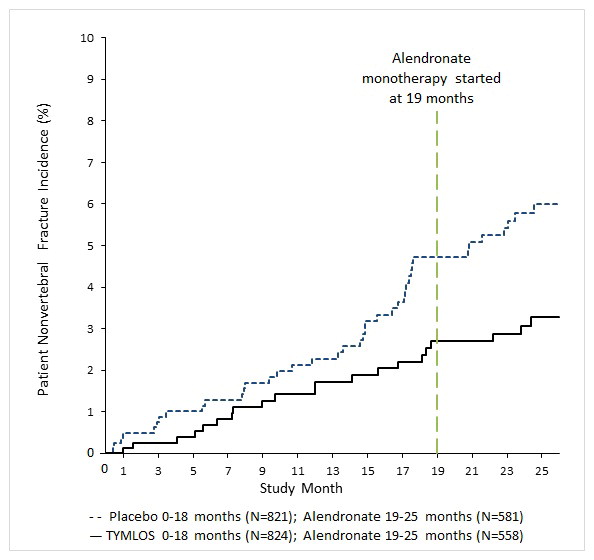
14 CLINICAL STUDIES14.1 Efficacy Study in Women with Postmenopausal Osteoporosis - The efficacy of TYMLOS for the treatment of postmenopausal osteoporosis was evaluated in Study 003 (NCT 01343004), an 18-month ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - TYMLOS (abaloparatide) injection is a clear and colorless solution, available as a pre-assembled single-patient-use disposable pen (NDC 70539-001-01) packaged in a cardboard ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Risk of Osteosarcoma - Advise patients that the active ingredient in TYMLOS ...
-
MEDICATION GUIDEMEDICATION GUIDE TYMLOS® (tim lows') (abaloparatide) injection, for subcutaneous use What is the most ...
-
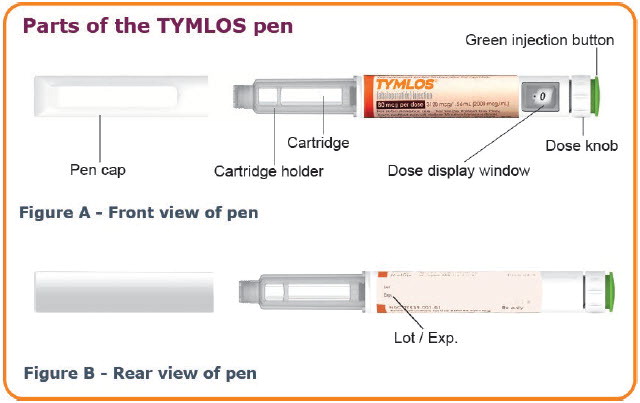
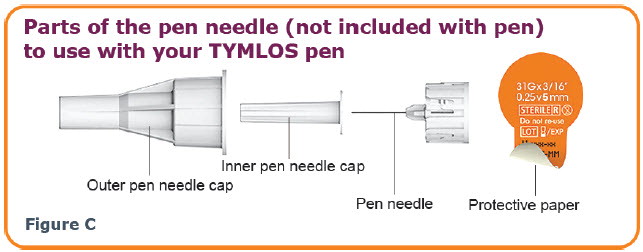
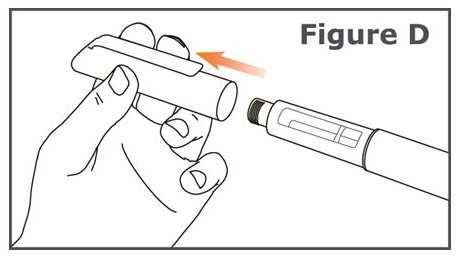
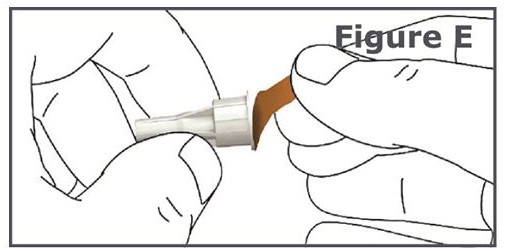
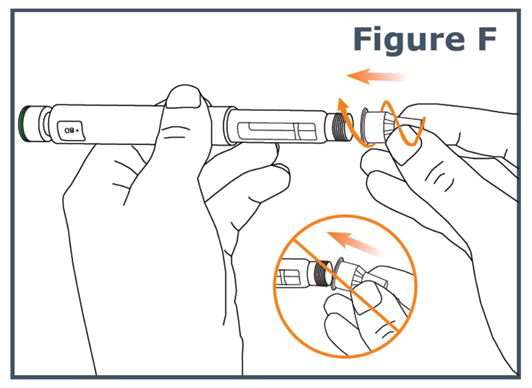
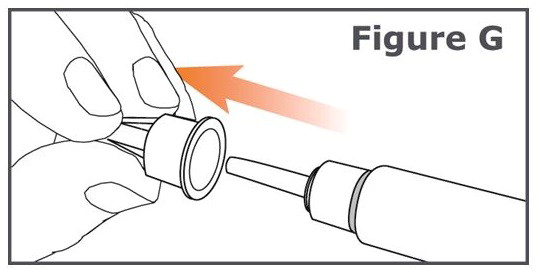
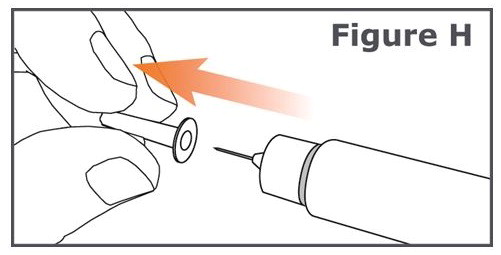
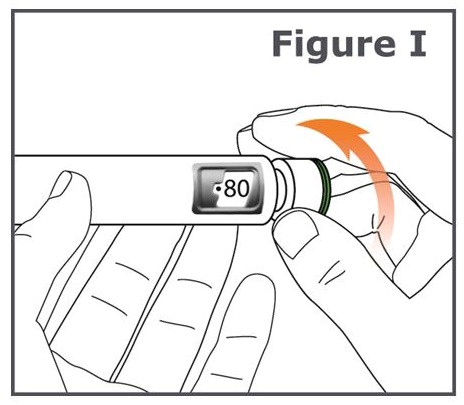
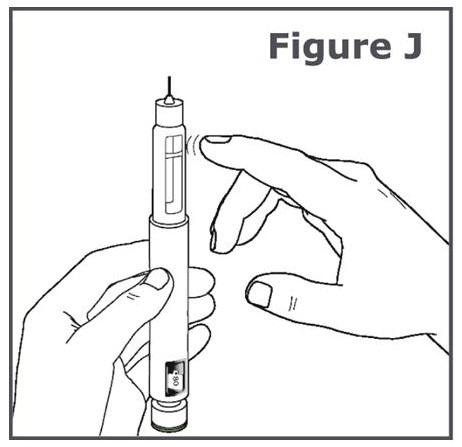
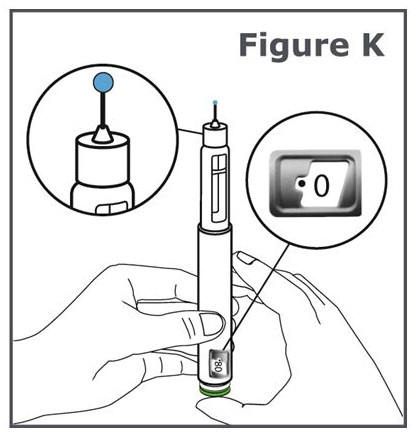
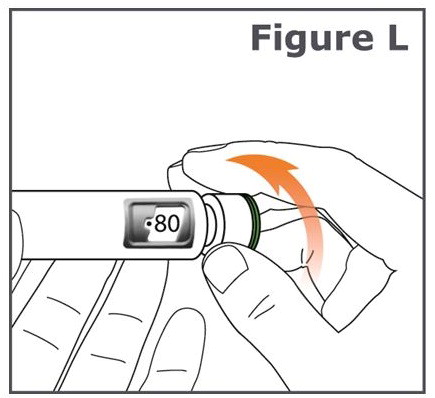
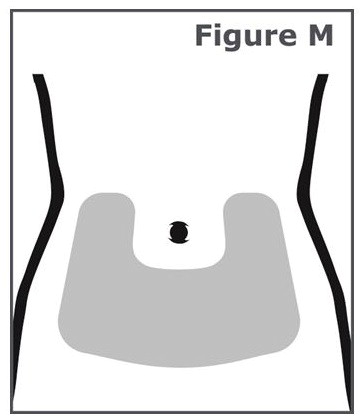
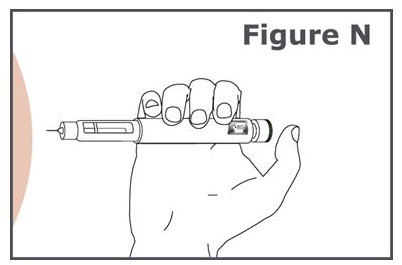
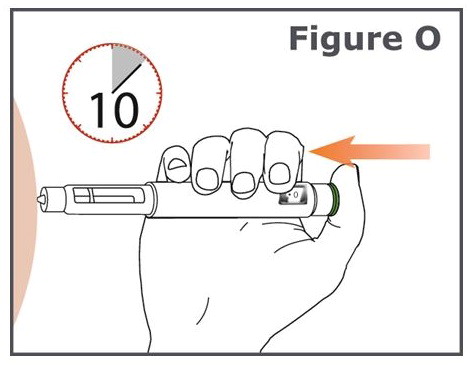
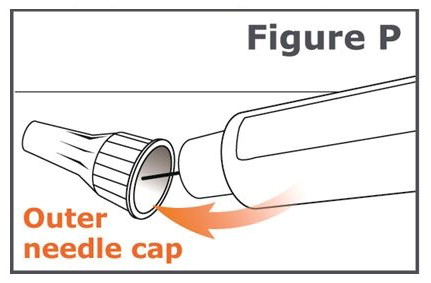
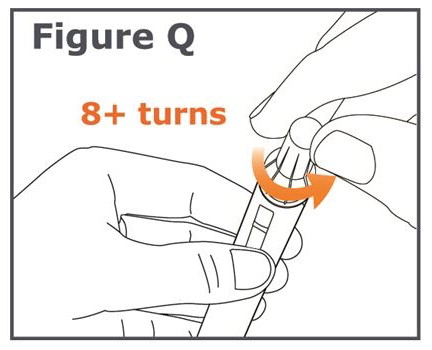
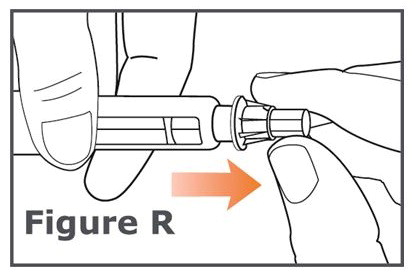
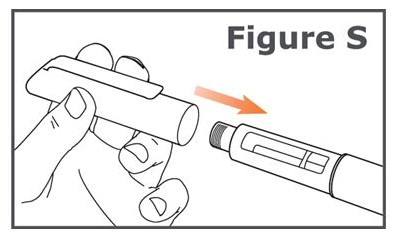
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE TYMLOS® (tim lows') (abaloparatide) injection, for subcutaneous use Instructions for Use - Read and follow this Instructions for ...
-
PRINCIPAL DISPLAY PANEL - 80 mcg Prefilled Pen LabelNDC 70539-001-01 - Rx only - Read Instructions for Use BEFORE injecting. Date of first use ________/________/________. Discard unused portion 30 days after first opening. TYMLOS® (abaloparatide ...
-
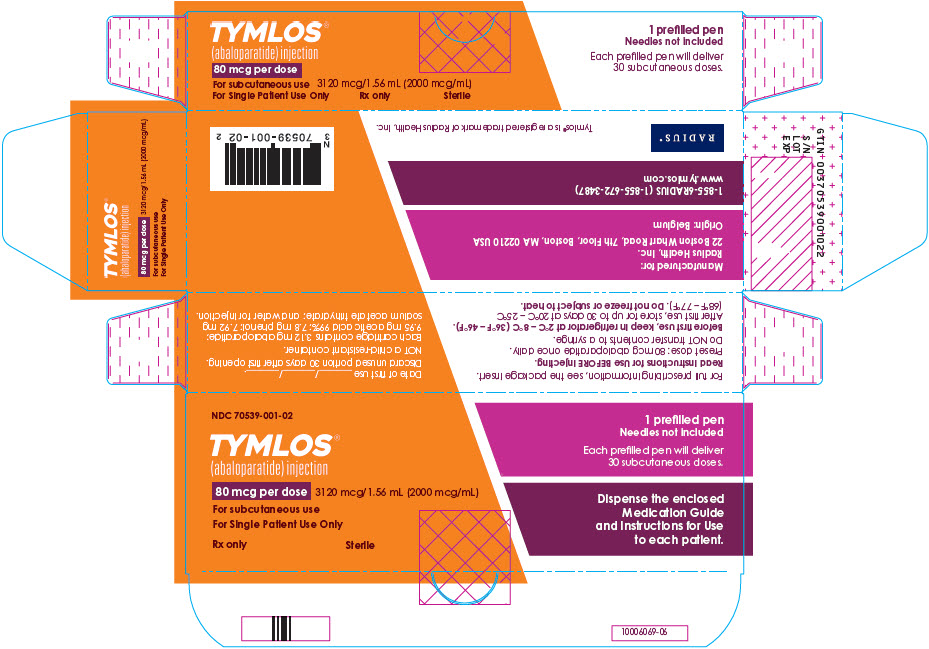
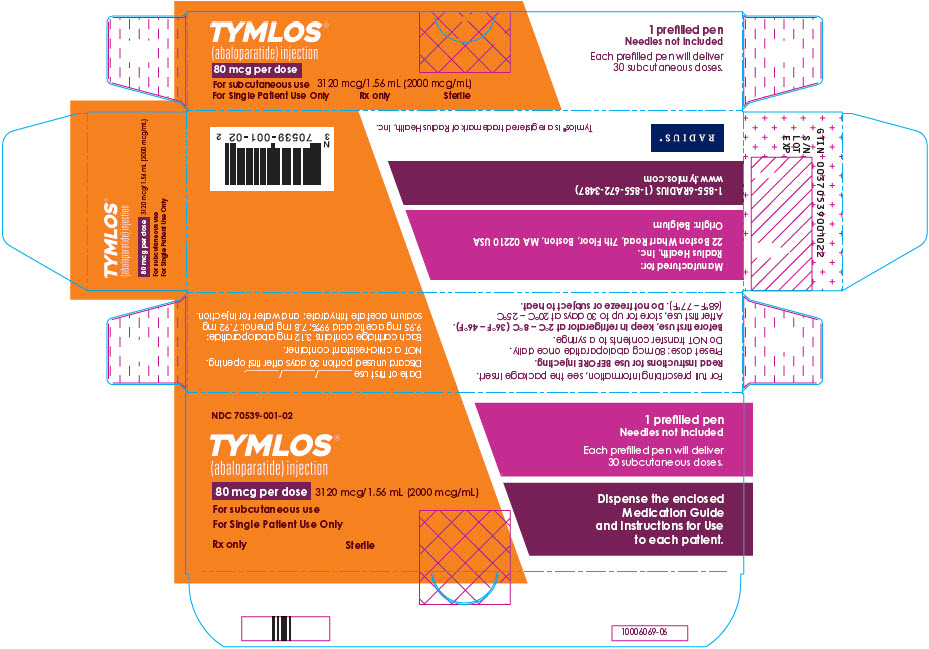
PRINCIPAL DISPLAY PANEL - 80 mcg Carton LabelNDC 70539-001-02 - TYMLOS® (abaloparatide) injection - 80 mcg per dose - 3120 mcg/1.56 mL (2000 mcg/mL) For subcutaneous use - For Single Patient Use Only - Rx only - Sterile - 1 prefilled pen - Needles not ...
-
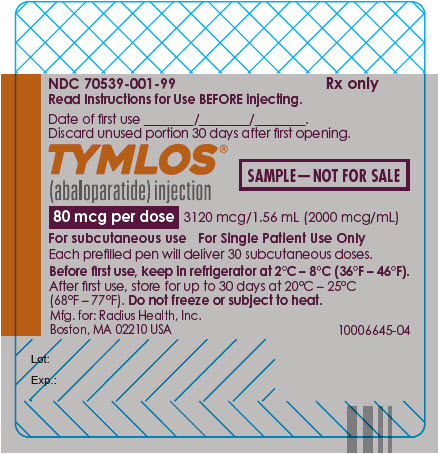
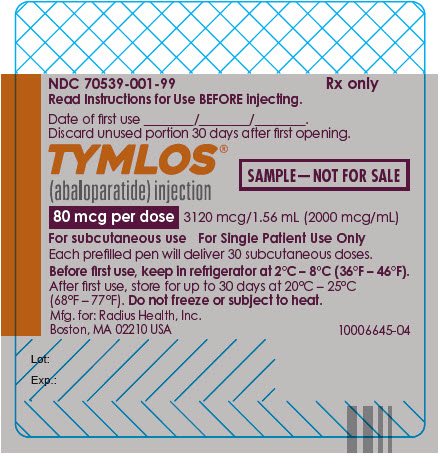
PRINCIPAL DISPLAY PANEL - Sample 80 mcg Prefilled Pen LabelNDC 70539-001-99 - Rx only - Read Instructions for Use BEFORE injecting. Date of first use ________/________/________. Discard unused portion 30 days after first opening. TYMLOS® (abaloparatide ...
-
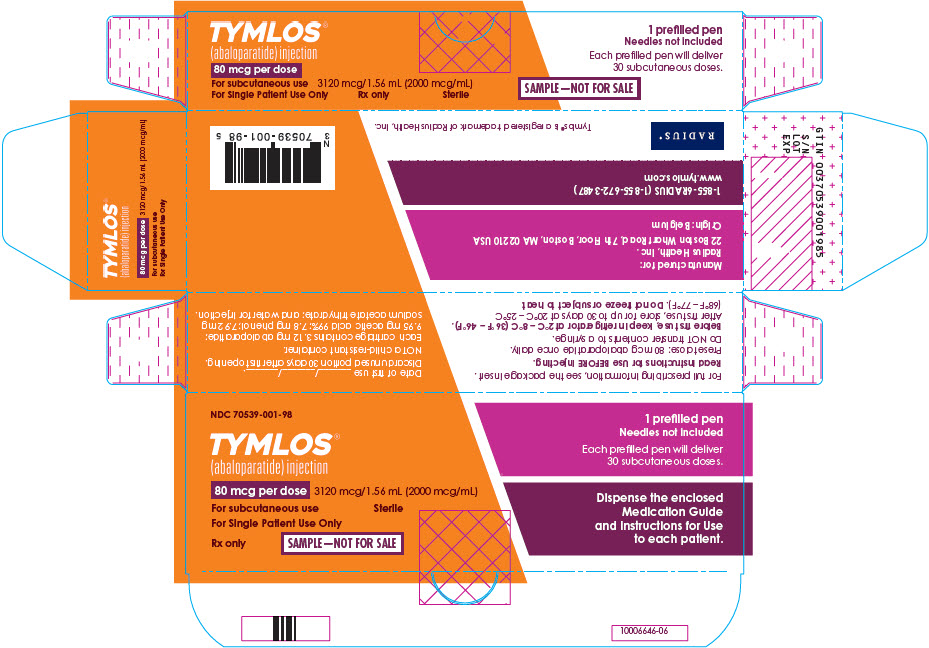
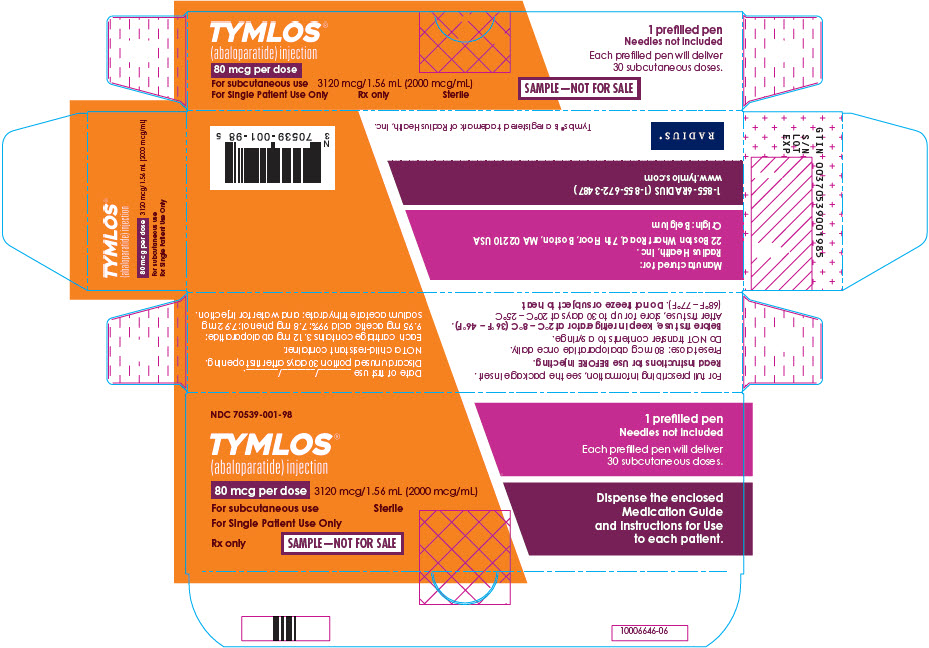
PRINCIPAL DISPLAY PANEL - Sample 80 mcg Carton LabelNDC 70539-001-98 - TYMLOS® (abaloparatide) injection - 80 mcg per dose - 3120 mcg/1.56 mL (2000 mcg/mL) For subcutaneous use - For Single Patient Use Only - Sterile - Rx only - SAMPLE—NOT FOR SALE - 1 ...
-
INGREDIENTS AND APPEARANCEProduct Information