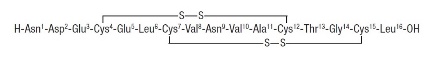Label: TRULANCE IMMEDIATE RELEASE- plecanatide tablet
- NDC Code(s): 65649-003-01, 65649-003-03, 65649-003-07, 65649-003-30
- Packager: Salix Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRULANCE safely and effectively. See full prescribing information for TRULANCE. TRULANCE - ®(plecanatide) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
- TRULANCE is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile mice, administration of a single oral dose of plecanatide caused deaths due to dehydration [see Contraindications (4), Use in Specific Populations (8.4)] .
- Avoid use of TRULANCE in patients 6 years to less than 18 years of age [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)] .
- The safety and effectiveness of TRULANCE have not been established in patients less than 18 years of age [see Use in Specific Populations (8.4)] .
-
1 INDICATIONS AND USAGETRULANCE is indicated in adults for the treatment of: chronic idiopathic constipation (CIC). irritable bowel syndrome with constipation (IBS-C).
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of TRULANCE for the treatment of CIC and IBS-C is 3 mg taken orally once daily. 2.2 Preparation and Administration Instructions - Take TRULANCE ...
-
3 DOSAGE FORMS AND STRENGTHSTRULANCE Tablets: 3 mg: white to off-white, plain, round tablet debossed with “SP” on one side and “3” for 3 mg on the other side.
-
4 CONTRAINDICATIONSTRULANCE is contraindicated in: Patients less than 6 years of age due to the risk of serious dehydration - [see - Warnings and Precautions (5.1), Use in Specific Populations ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Serious Dehydration in Pediatric Patients - TRULANCE is contraindicated in patients less than 6 years of age. The safety and effectiveness of TRULANCE in patients less than 18 years ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Plecanatide and its active metabolite are negligibly absorbed systemically following oral administration - [see - Clinical Pharmacology (12.3)] and ...
-
11 DESCRIPTIONTRULANCE (plecanatide) is a guanylate cyclase-C (GC-C) agonist. Plecanatide is a 16 amino acid peptide with the following chemical name: L-Leucine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Plecanatide is a structural analog of human uroguanylin, and similarly to uroguanylin, plecanatide functions as a guanylate cyclase-C (GC-C) agonist. Both plecanatide ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of plecanatide was assessed in 2-year carcinogenicity studies in mice and rats ...
-
14 CLINICAL STUDIES14.1 Chronic Idiopathic Constipation (CIC) The efficacy of TRULANCE for the management of symptoms of CIC was established in two 12-week, double-blind, placebo-controlled, randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTRULANCE tablets are packaged in a white, opaque, high-density polyethylene round bottle with a screw-top polypropylene child-resistant cap and heat-activated induction seal. Each bottle ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Advise patients: Diarrhea - To stop TRULANCE and contact their healthcare provider if they experience severe ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Salix Pharmaceuticals, a division of - Bausch Health US, LLC - Bridgewater, NJ 08807 USA - Patented. See https://patents.salix.com for US patent information. TRULANCE ...
-
MEDICATION GUIDEMedication Guide - TRULANCE - ®(TROO lans) (plecanatide) tablets, for oral use - What is the most important information I should know about TRULANCE? Do not give ...
-
PRINCIPAL DISPLAY PANEL - 3 mg Tablet LabelNDC65649-003-30 - Rx only - 30 Tablets - Trulance - ® (plecanatide) tablets - 3 mg - ATTENTION PHARMACIST: Dispense the accompanying - Medication Guide to each patient. Keep ...
-
INGREDIENTS AND APPEARANCEProduct Information