Label: TROGARZO- ibalizumab injection, solution
- NDC Code(s): 62064-122-01, 62064-122-02
- Packager: Theratechnologies Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TROGARZO safely and effectively. See full prescribing information for TROGARZO. TROGARZO® (ibalizumab-uiyk) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETROGARZO, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - The recommended dosage regimen is a single loading dose of 2,000 mg followed by a maintenance dose of 800 mg every 2 weeks administered as a diluted intravenous infusion ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection: 200 mg/1.33 mL (150 mg/mL) colorless to slightly yellow and clear to slightly opalescent solution with no visible particles in a single-dose vial.
-
4 CONTRAINDICATIONS
TROGARZO is contraindicated in patients with a prior hypersensitivity reaction to TROGARZO or any components of the product [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Including Infusion-Related and Anaphylactic Reactions - Hypersensitivity reactions including infusion-related reactions and anaphylactic reactions have been reported ...
-
6 ADVERSE REACTIONS
The following adverse drug reactions are discussed in other sections of the labeling: Immune Reconstitution Inflammatory Syndrome [see Warnings and Precautions (5.2)] 6.1 Clinical Trial ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiretrovirals during pregnancy. This registry does ...
-
11 DESCRIPTION
TROGARZO is a CD4-directed post-attachment HIV-1 inhibitor. Ibalizumab-uiyk is a CD4 domain 2-directed humanized monoclonal antibody of immunoglobulin G (IgG) isotype 4 with a molecular weight of ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Ibalizumab-uiyk is an HIV-1 antiretroviral drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - A clear trend was identified between exposure and response rate ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis, mutagenesis, and fertility studies with ibalizumab-uiyk have not been conducted.
-
14 CLINICAL STUDIES
Trial TMB-301: Trial TMB-301 was a single arm, multicenter clinical trial conducted in 40 heavily treatment-experienced HIV-infected subjects with multidrug resistant HIV-1. Subjects were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TROGARZO (ibalizumab-uiyk) injection is a sterile colorless to slightly yellow and clear to slightly opalescent solution with no visible particles for intravenous administration (by IV ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Hypersensitivity - Advise patients of the risk of hypersensitivity reactions including anaphylaxis. Instruct ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - TROGARZO® (tro-gar-zo) (ibalizumab-uiyk) injection - What is TROGARZO? TROGARZO is a prescription medicine that is used with other antiretroviral medicines to treat Human ...
-
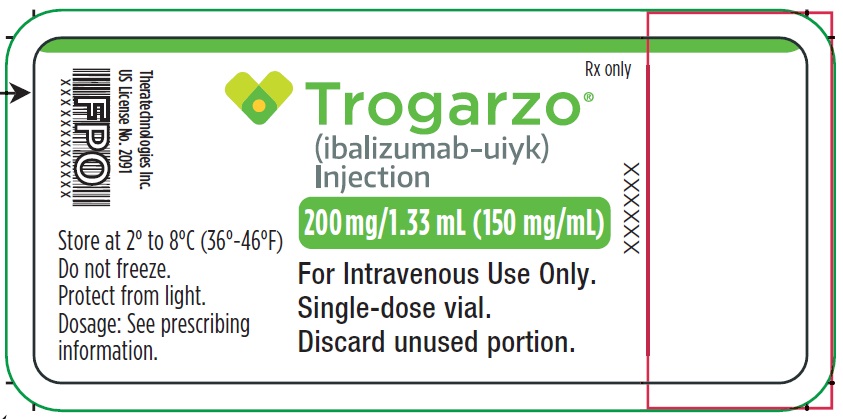
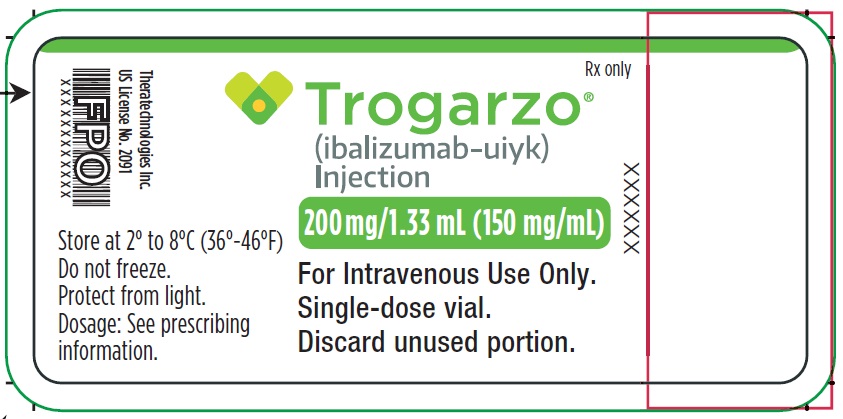
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Trogarzo Vial Label - Rx only - Trogarzo® (ibalizumab-uiyk) Injection - 200 mg/1.33 mL (150 mg/mL) For Intravenous Use Only. Single-dose vial. Discard unused portion. Store ...
-
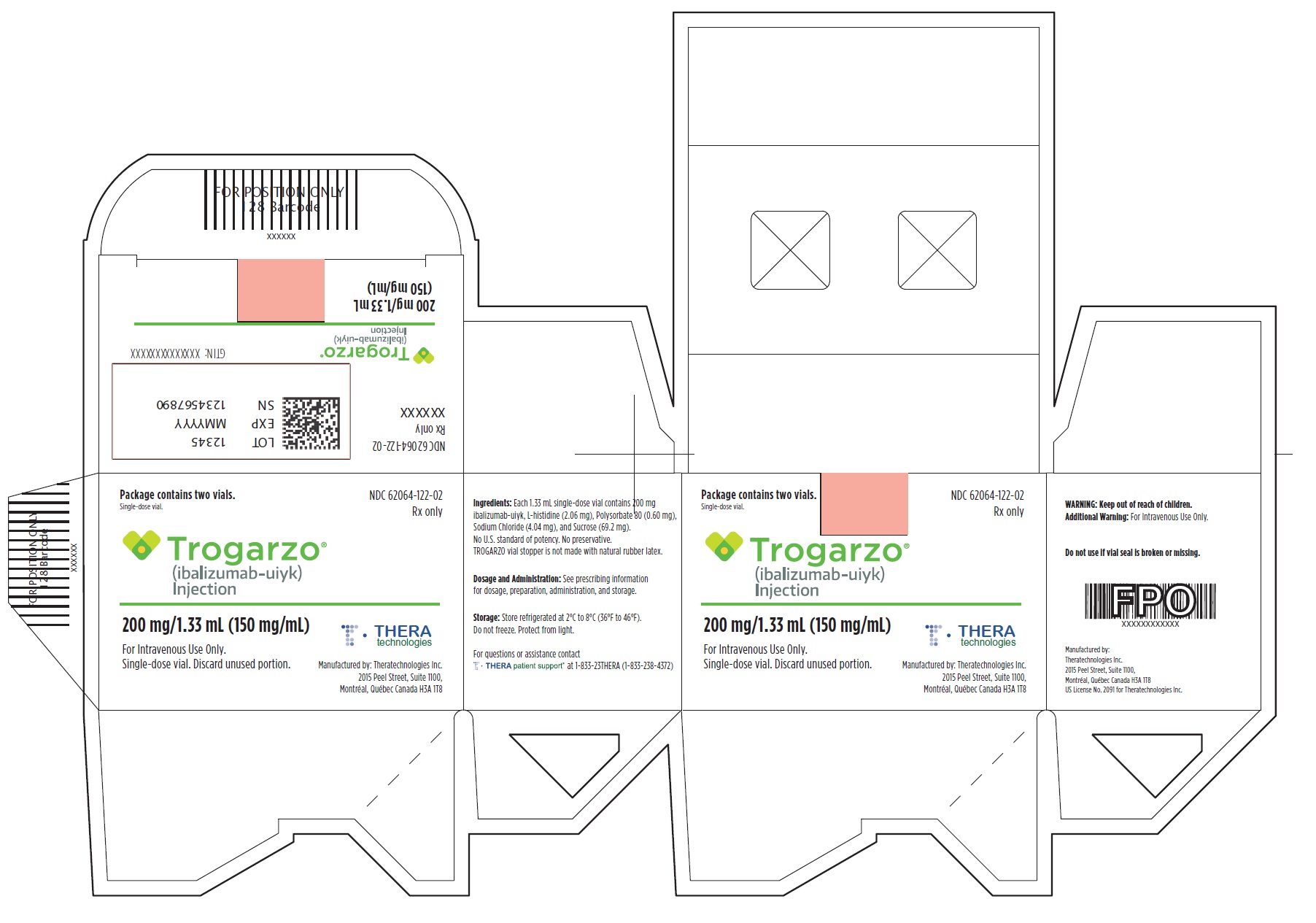
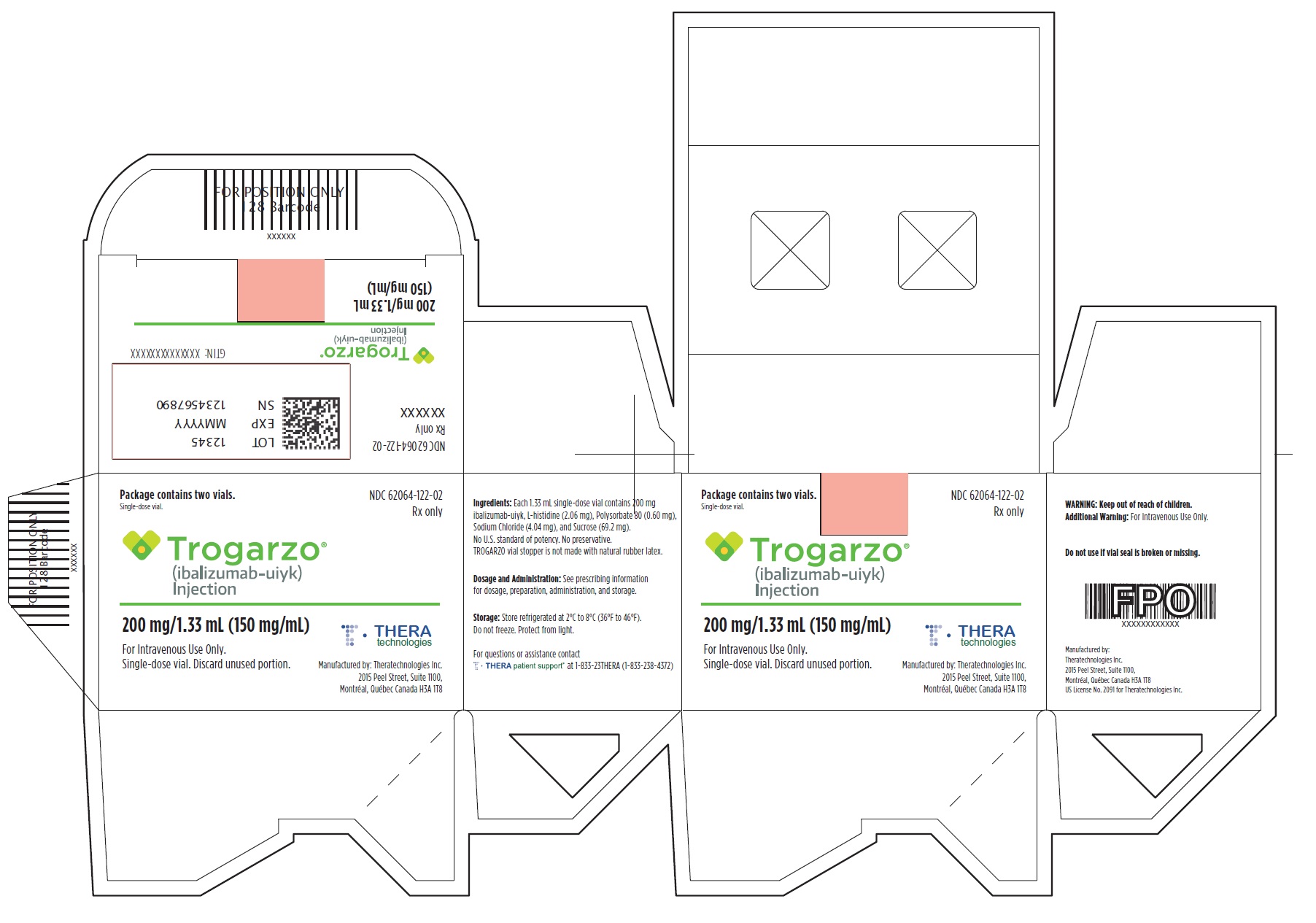
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Trogarzo Carton Label - Package contains two vials. Single-dose vial. NDC 62064-122-02 - Rx only - Trogarzo® (ibalizumab-uiyk) Injection - 200 mg/1.33 mL (150 mg/mL) For ...
-
INGREDIENTS AND APPEARANCEProduct Information


 at 1-833-23THERA (1-833-238-4372).
at 1-833-23THERA (1-833-238-4372).