Label: TRISENOX- arsenic trioxide injection, solution
- NDC Code(s): 63459-601-06, 63459-601-11
- Packager: Cephalon, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRISENOX safely and effectively. See full prescribing information for TRISENOX. TRISENOX® (arsenic trioxide) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DIFFERENTIATION SYNDROME, CARDIAC CONDUCTION ABNORMALITIES AND ENCEPHALOPATHY INCLUDING WERNICKE'S
Differentiation Syndrome: Patients with acute promyelocytic leukemia (APL) treated with TRISENOX have experienced differentiation syndrome, which may be life-threatening or fatal. Signs and symptoms may include unexplained fever, dyspnea, hypoxia, acute respiratory distress, pulmonary infiltrates, pleural or pericardial effusions, weight gain, peripheral edema, hypotension, renal insufficiency, hepatopathy, and multi-organ dysfunction, in the presence or absence of leukocytosis. If differentiation syndrome is suspected, immediately initiate high-dose corticosteroids and hemodynamic monitoring until resolution. Temporarily withhold TRISENOX [see Dosage and Administration (2.3), Warnings and Precautions (5.1)].
Cardiac Conduction Abnormalities: TRISENOX can cause QTc interval prolongation, complete atrioventricular block and torsade de pointes, which can be fatal. Before administering TRISENOX, assess the QTc interval, correct electrolyte abnormalities, and consider discontinuing drugs known to prolong QTc interval. Do not administer TRISENOX to patients with a ventricular arrhythmia or prolonged QTc interval. Withhold TRISENOX until resolution and resume at reduced dose for QTc prolongation [see Dosage and Administration (2.3), Warnings and Precautions (5.2)].
Encephalopathy: Serious encephalopathy, including Wernicke's, has occurred with TRISENOX. Wernicke's is a neurologic emergency. Consider testing thiamine levels in patients at risk for thiamine deficiency. Administer parenteral thiamine in patients with or at risk for thiamine deficiency. Monitor patients for neurological symptoms and nutritional status while receiving TRISENOX. If Wernicke's encephalopathy is suspected, immediately interrupt TRISENOX and initiate parenteral thiamine. Monitor until symptoms resolve or improve and thiamine levels normalize [see Warnings and Precautions (5.3)].
Close -
1 INDICATIONS AND USAGE
1.1 Newly-Diagnosed Low-Risk APL - TRISENOX is indicated in combination with tretinoin for treatment of adults with newly-diagnosed low-risk acute promyelocytic leukemia (APL) whose APL is ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Newly-Diagnosed Low-Risk Acute Promyelocytic Leukemia (APL) A treatment course for patients with newly-diagnosed low-risk APL consists of 1 induction cycle and 4 ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection: 12 mg/6 mL (2 mg/mL) arsenic trioxide clear solution in a single-dose vial
-
4 CONTRAINDICATIONS
TRISENOX is contraindicated in patients with hypersensitivity to arsenic.
-
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome - Differentiation syndrome, which may be life-threatening or fatal, has been observed in patients with acute promyelocytic leukemia (APL) treated with TRISENOX. In ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling: Differentiation Syndrome [see Warnings and Precautions (5.1)] Cardiac Conduction Abnormalities ...
-
7 DRUG INTERACTIONS
Drugs That Can Prolong the QT/QTc Interval - Concomitant use of these drugs and TRISENOX may increase the risk of serious QT/QTc interval prolongation [see Warnings and Precautions (5.1)] ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Based on the mechanism of action [see Clinical Pharmacology (12.1)] and findings in animal studies, TRISENOX can cause fetal harm when administered to a pregnant ...
-
10 OVERDOSAGE
Manifestations - Manifestations of TRISENOX (arsenic trioxide) overdosage include convulsions, muscle weakness, and confusion. Management - For symptoms of TRISENOX (arsenic trioxide) overdosage ...
-
11 DESCRIPTION
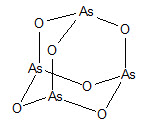
TRISENOX is a sterile injectable solution of arsenic trioxide. The molecular formula of arsenic trioxide in the solid state is As2O3, with a molecular weight of 197.8 and the following structural ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism of action of TRISENOX is not completely understood. Arsenic trioxide causes morphological changes and DNA fragmentation characteristic of apoptosis in ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted with TRISENOX [see Warnings and Precautions (5.6)]. Arsenic trioxide and trivalent ...
-
14 CLINICAL STUDIES
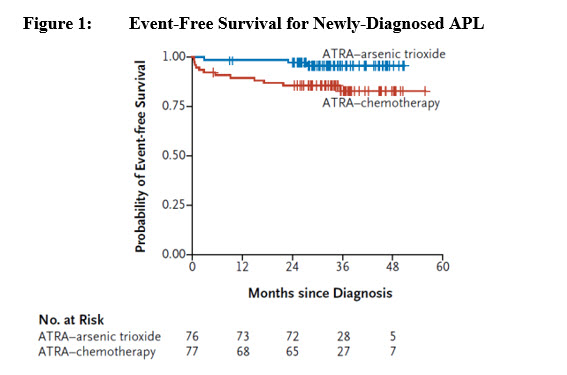
14.1 Newly-Diagnosed Low-Risk APL - TRISENOX in combination with tretinoin was investigated in Study APL0406 (NCT00482833), a multicenter, randomized, open-label trial in patients with ...
-
15 REFERENCES
“OSHA Hazardous Drugs.” OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - TRISENOX (arsenic trioxide) injection is supplied as a sterile, clear, colorless solution in 10 mL glass, single-dose vials. NDC 63459-601-06: 12 mg/6 mL (2 mg/mL) vial in packages ...
-
17 PATIENT COUNSELING INFORMATION
Differentiation Syndrome - Advise patients that symptoms of APL differentiation syndrome include fever, sudden weight gain, dizziness/lightheadedness, labored breathing, and accumulation of ...
-
Package/Label Display PanelNDC 63459-601-06 - Trisenox® (arsenic trioxide) injection - 12 mg/6 mL (2 mg/mL) 10 x 6 mL - Single-Dose Vial – Discard Unused Portion - For Intravenous Use Only - 10 x 6 mL - Single-Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information