Label: TRIPTODUR- triptorelin kit
- NDC Code(s): 24338-150-20
- Packager: Azurity Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRIPTODUR - ®safely and effectively. See full prescribing information for TRIPTODUR. TRIPTODUR (triptorelin) for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETRIPTODUR is indicated for the treatment of pediatric patients 2 years of age and older with central precocious puberty (CPP).
-
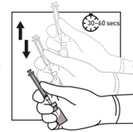
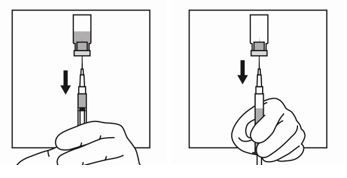
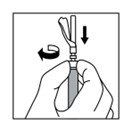
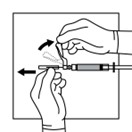
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - TRIPTODUR must only be administered by a healthcare provider. The dosage of TRIPTODUR is 22.5 mg reconstituted with accompanying diluent (Sterile Water) 2 mL, and ...
-
3 DOSAGE FORMS AND STRENGTHSFor extended-release injectable suspension: 22.5 mg of triptorelin as a lyophilized white to slightly yellow powder cake in a single-dose vial for reconstitution with the co-packaged 2 mL of ...
-
4 CONTRAINDICATIONSHypersensitivity: TRIPTODUR is contraindicated in individuals with a known hypersensitivity to triptorelin, any other component of the product, or other GnRH agonists or GnRH - [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Initial Rise of Gonadotropins and Sex Steroid Levels - During the early phase of initial therapy or after subsequent doses, gonadotropins and sex steroids may rise above baseline because of a ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described here and elsewhere in the label: Initial Rise of Gonadotropins and Sex Steroid Levels - [see - Warnings and Precautions (5.1) ...
-
7 DRUG INTERACTIONS7.1 Drug-Drug Interactions - Results of - in vitro studies show that drug-drug interactions with triptorelin are unlikely - [see Clinical Pharmacology (12.3)]. However, in the absence of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - TRIPTODUR is contraindicated in women who are pregnant - [see - Contraindications (4)] since expected hormonal changes that occur with TRIPTODUR ...
-
10 OVERDOSAGEThere is no experience with overdosage in clinical trials of triptorelin. If overdosage occurs, therapy should be discontinued and appropriate supportive and symptomatic treatment ...
-
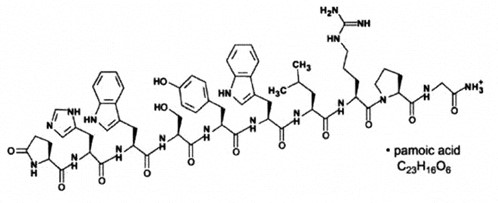
11 DESCRIPTIONTRIPTODUR contains the pamoate salt of triptorelin, a synthetic decapeptide analog of naturally occurring gonadotropin-releasing hormone (GnRH or LHRH). The chemical name of triptorelin pamoate is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Triptorelin is a GnRH agonist. 12.2 Pharmacodynamics - Following the first administration, there is a transient surge in circulating levels of LH, FSH, testosterone ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis was evaluated in an 18-month study in mice and a 24-month study in rats. In rats, triptorelin doses of 120, 600, and ...
-
14 CLINICAL STUDIESIn a single-arm open-label study, 44 children 2 to 9 years of age with CPP, 39 females and 5 males, all naïve to previous GnRH agonist treatment, were administered TRIPTODUR 22.5 mg at a dosing ...
-
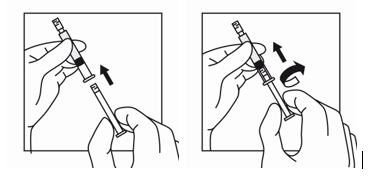
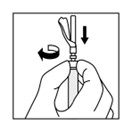
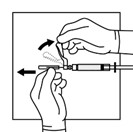
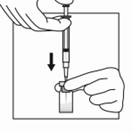
16 HOW SUPPLIED/STORAGE AND HANDLINGEach TRIPTODUR 22.5 mg single-use kit (NDC 24338-150-20) contains: One single-dose vial of TRIPTODUR 22.5 mg (NDC 24338-150-01) with a Flip-Off seal containing sterile lyophilized white to ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Medication Guide). Hypersensitivity Reactions - Inform caregivers that anaphylactic shock ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Azurity Pharmaceuticals, Inc. Woburn, MA 01801 - Manufactured by: Debiopharm Research & Manufacturing SA - CH-1920 Martigny, Switzerland - TRIPTODUR is a ...
-
MEDICATION GUIDEMEDICATION GUIDE - TRIPTODUR - ®[TRIP-toe-der] (triptorelin) for extended-release injectable suspension, for intramuscular use - This Medication ...
-
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 24338-150-20 - Rx Only - Triptodur - ® (triptorelin) for extended-release injectable suspension - 22.5 mg - KIT - 22.5 mg every 24 weeks - FOR INTRAMUSCULAR ...
-
INGREDIENTS AND APPEARANCEProduct Information