Label: TPOXX- tecovirimat monohydrate injection, solution, concentrate
TPOXX- tecovirimat monohydrate capsule
- NDC Code(s): 50072-010-01, 50072-010-30, 50072-200-42
- Packager: SIGA Technologies, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TPOXX® safely and effectively. See full prescribing information for TPOXX. TPOXX (tecovirimat) capsules, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Treatment of Human Smallpox Disease - TPOXX® is indicated for the treatment of human smallpox disease caused by variola virus in adults and pediatric patients weighing at least 3 ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosing Instructions - It is recommended that patients 13 kg and above initiate oral treatment with TPOXX capsules if possible. If patients are unable to take oral TPOXX capsules or ...
-
3 DOSAGE FORMS AND STRENGTHSTPOXX Capsules - TPOXX capsules are hard gelatin with an opaque orange body imprinted in white ink with “SIGA” followed by the SIGA logo followed by “®”, and an opaque black cap imprinted in white ...
-
4 CONTRAINDICATIONSTPOXX Capsules: None. TPOXX Injection: The excipient hydroxypropyl-β-cyclodextrin is eliminated through glomerular filtration. Therefore, TPOXX Injection is contraindicated in patients with ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia When Co-Administered with Repaglinide - Co-administration of repaglinide and tecovirimat may cause mild to moderate hypoglycemia. Monitor blood glucose and monitor for ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Effect of TPOXX on Other Drugs - Tecovirimat is a weak inducer of cytochrome P450 (CYP)3A and a weak inhibitor of CYP2C8 and CYP2C19. However, the effects are not expected to be clinically ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on the use of tecovirimat in pregnant individuals to evaluate for a drug-associated risk of major birth defects, miscarriage, and other ...
-
10 OVERDOSAGEThere is no clinical experience with overdosage of TPOXX. In case of overdosage, monitor patients for any signs or symptoms of adverse effects. Hemodialysis will not significantly remove TPOXX ...
-
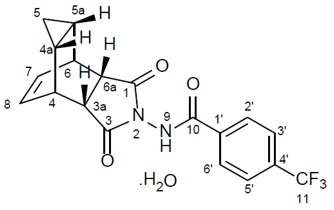
11 DESCRIPTIONTPOXX capsules and TPOXX injection contains tecovirimat, an inhibitor of the orthopoxvirus VP37 envelope wrapping protein. TPOXX (tecovirimat) capsules, for oral use are immediate release capsules ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tecovirimat is an antiviral drug against variola (smallpox) virus [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Cardiac Electrophysiology - TPOXX does not ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis and Mutagenesis - Carcinogenicity studies have not been conducted with tecovirimat. Tecovirimat was not genotoxic in in ...
-
14 CLINICAL STUDIESOverview - The effectiveness of TPOXX for treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible, and inducing ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTPOXX Capsule - How Supplied - Each TPOXX capsule contains 200 mg of tecovirimat. TPOXX capsules are hard gelatin with an opaque orange body imprinted in white ink with “SIGA” followed by the ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Efficacy Based on Animal Models Alone - Inform patients that the efficacy of TPOXX is ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 05/2022 - PATIENT INFORMATION - TPOXX (Tē-Pox or ...
-
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - TPOXX (Tē-Pox or Tee-pahx) (tecovirimat) capsules, for oral use - This Instructions for Use contains information on how to prepare and give ...
-
PRINCIPAL DISPLAY PANEL - NDC: 50072-200-42 - 200 mg Capsule 42-count Bottle Label

-
PRINCIPAL DISPLAY PANEL - NDC: 50072-010-30 - 200 mg/20 mL (10 mg/mL Vial Label)

-
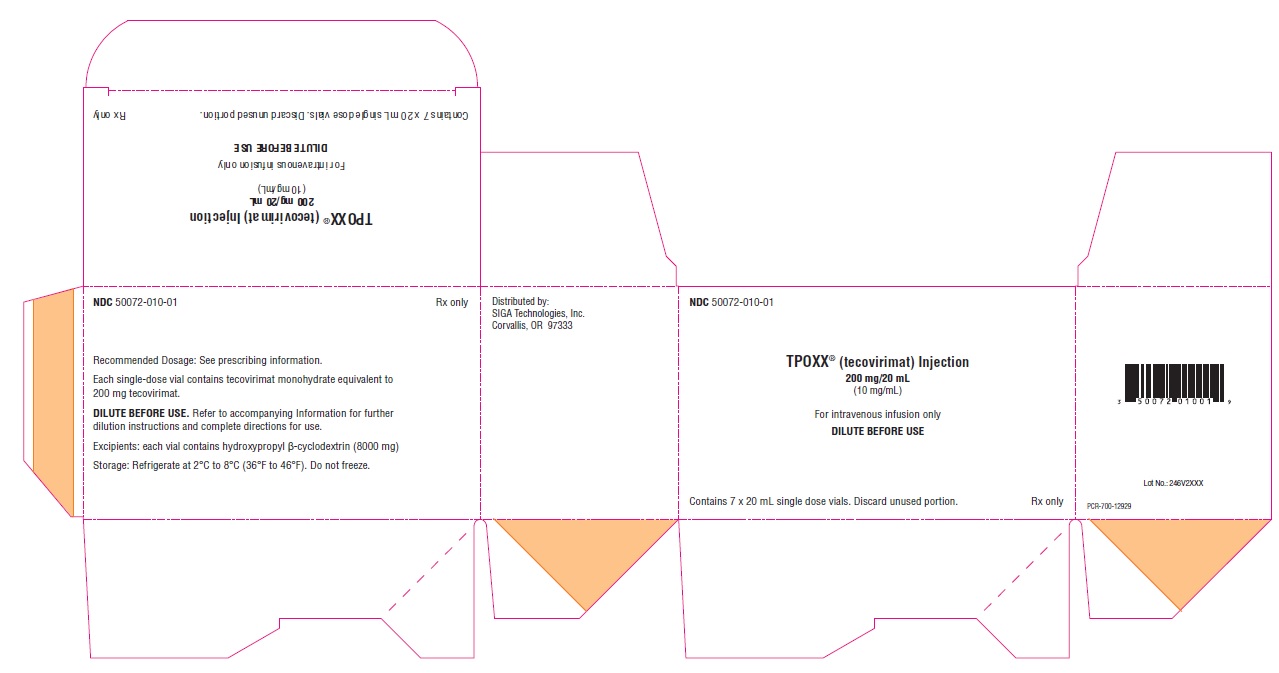
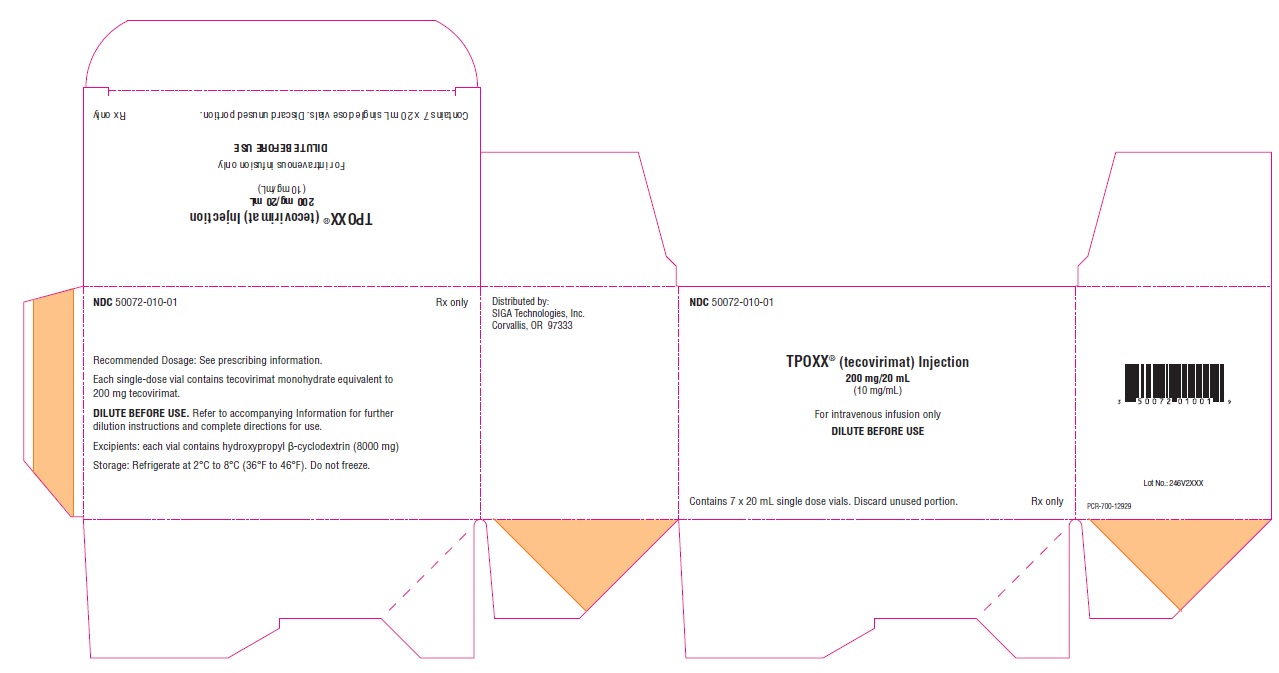
PRINCIPAL DISPLAY PANEL - NDC: 50072-010-01 - 7 Vial Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information