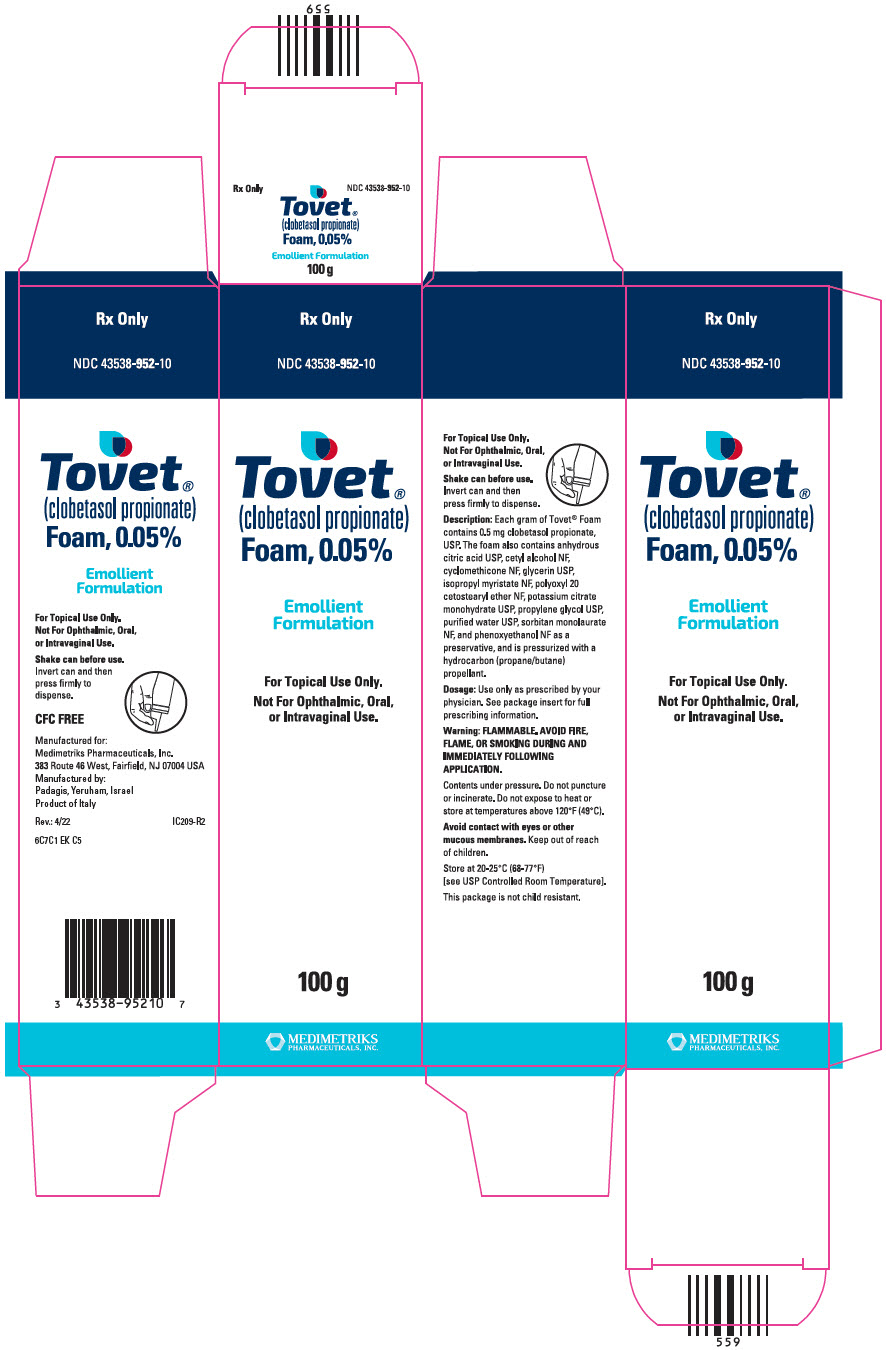
Label: TOVET (EMOLLIENT FORMULATION)- clobetasol propionate aerosol, foam
- NDC Code(s): 43538-952-10
- Packager: Medimetriks Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TOVET (CLOBETASOL PROPIONATE) FOAM (EMULSION) safely and effectively. See full prescribing information for TOVET (CLOBETASOL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETovet Foam is indicated for the treatment of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 12 years and older.
-
2 DOSAGE AND ADMINISTRATIONApply a thin layer of Tovet Foam to the affected area(s) twice daily, morning and evening, for up to 2 consecutive weeks; therapy should be discontinued when control has been achieved. The ...
-
3 DOSAGE FORMS AND STRENGTHSTovet (clobetasol propionate) Foam, 0.05% (Emulsion) contains 0.5 mg of clobetasol propionate, USP per gram.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Effects on Endocrine System - Clobetasol propionate foam, 0.05% (emulsion) has been shown to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Systemic absorption of clobetasol ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Effects on Endocrine System [see Warnings and Precautions (5.1)] Ophthalmic Adverse Reactions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on Tovet Foam use in pregnant women to inform of a drug associated risk for adverse developmental outcomes. Published data report a ...
-
10 OVERDOSAGETopically applied Tovet Foam can be absorbed in sufficient amounts to produce systemic effects.
-
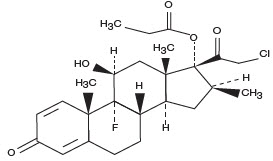
11 DESCRIPTIONTovet (clobetasol propionate) Foam, 0.05% (Emulsion) is a white to off-white petrolatum-based emulsion aerosol foam containing the active ingredient clobetasol propionate, USP, a synthetic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of clobetasol propionate foam, 0.05% (emulsion ...
-
14 CLINICAL STUDIESIn a randomized trial of subjects 12 years and older with moderate to severe atopic dermatitis, 251 subjects were treated with clobetasol propionate foam, 0.05% (emulsion) and 126 subjects were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Tovet® (clobetasol propionate) Foam, 0.05% (Emulsion) contains 0.5 mg of clobetasol propionate, USP per gram. The white emulsion aerosol foam is available as ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (Patient Information) Effects on Endocrine System - Tovet Foam may cause HPA axis suppression. Advise patients that use of topical corticosteroids, including ...
-
SPL UNCLASSIFIED SECTIONRx Only - Manufactured for: Medimetriks Pharmaceuticals, Inc., 383 Route 46 West, Fairfield, NJ 07004 USA - Manufactured by: Padagis, Yeruham, Israel - Rev.: 4/22 - IP052-R1 - 6C700 EK J4
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Tovet® (clobetasol propionate) Foam, 0.05% (Emollient Formulation) IMPORTANT: For skin use only. Do not get Tovet Foam in your eyes, mouth, or vagina. What is ...
-
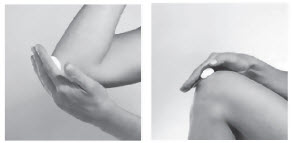
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - Tovet® (clobetasol propionate) Foam, 0.05% (Emollient Formulation) Important information: Tovet Foam is for use on the skin only. Do not get Tovet Foam in your eyes ...
-
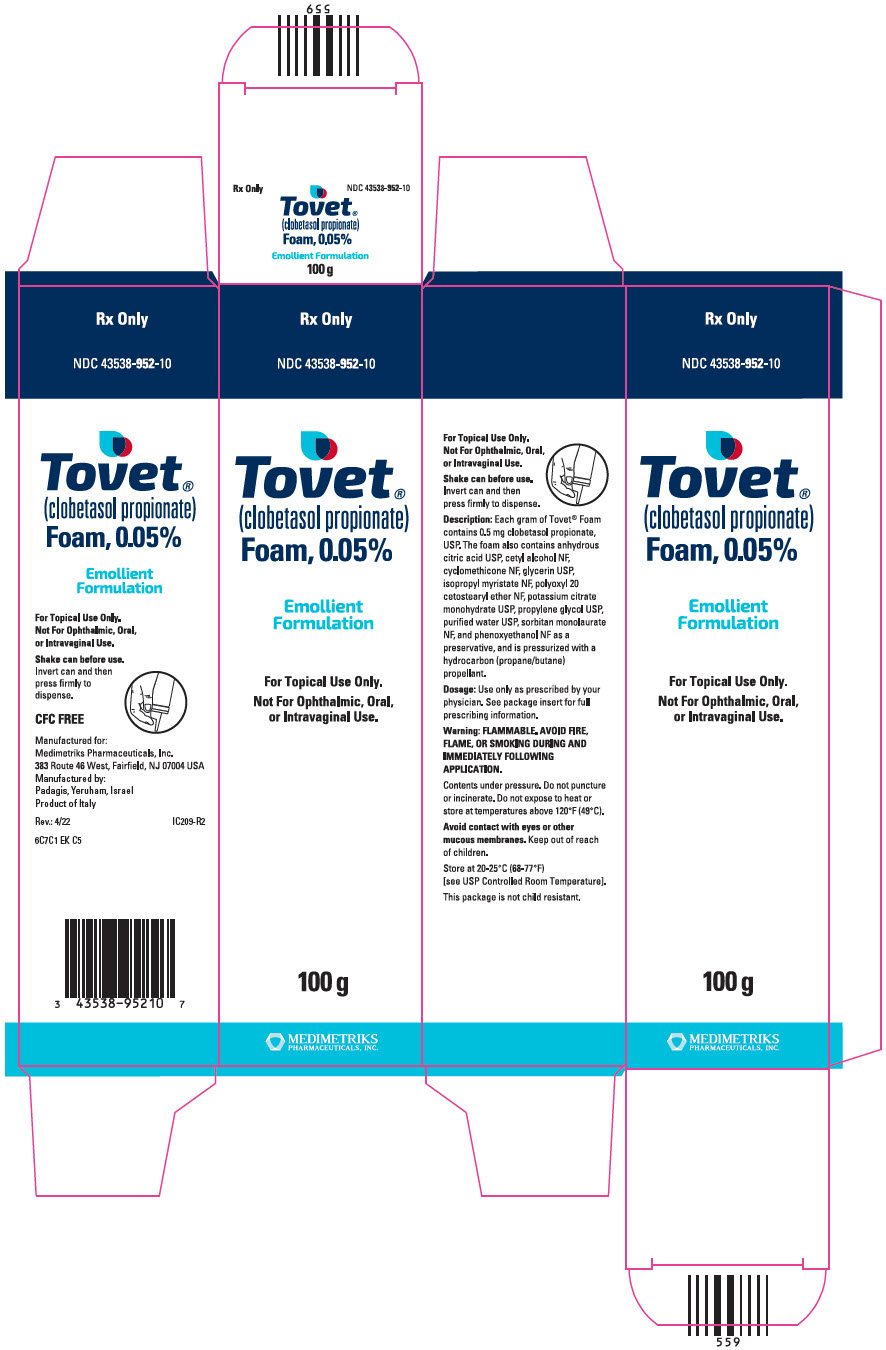
PRINCIPAL DISPLAY PANEL - 100 g Canister CartonRx Only - NDC 43538-952-10 - Tovet® (clobetasol propionate) Foam, 0.05% Emollient - Formulation - For Topical Use Only. Not For Ophthalmic, Oral, or Intravaginal Use. 100 g - MEDIMETRIKS ...
-
INGREDIENTS AND APPEARANCEProduct Information