Label: TOLSURA- itraconazole capsule, gelatin coated
- NDC Code(s): 51862-462-08, 51862-462-60, 51862-462-88
- Packager: Mayne Pharma Commercial LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TOLSURA® safely and effectively. See full prescribing information for TOLSURA®. TOLSURA® (itraconazole capsules), for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)Congestive Heart Failure - TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative ...
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
-
Congestive Heart Failure
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Close-
Drug Interactions
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs [see Contraindications (4.1) and Drug Interactions (7.1)]
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eliglustat is contraindicated in subjects that are poor or intermediate metabolizers of CYP2D6 and in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia [see Contraindications (4.1), Warnings and Precautions (5.5) and Drug Interactions (7.1)].
-
Congestive Heart Failure
-
1 INDICATIONS AND USAGETOLSURA is indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients: Blastomycosis, pulmonary and ...
-
2 DOSAGE AND ADMINISTRATIONTOLSURA must be administered with food. TOLSURA capsules must be swallowed whole. Do not chew, crush or break TOLSURA capsules. Table 1 below describes the recommended dosage for TOLSURA. Table ...
-
3 DOSAGE FORMS AND STRENGTHSTOLSURA (itraconazole capsules) is available in a size 1, hard gelatin capsules with light blue cap and white body, imprinted with "i-65" in black on the cap and containing 65 mg of ...
-
4 CONTRAINDICATIONS4.1 Drug Interactions - Co-administration of certain drugs that are metabolized by human CYP3A4 substrates are contraindicated with TOLSURA because plasma concentrations of such drugs are ...
-
5 WARNINGS AND PRECAUTIONS5.1 Congestive Heart Failure - TOLSURA can cause or exacerbate congestive heart failure (CHF) [see Boxed Warning and Adverse Reactions (6.1)]. For patients with evidence of ventricular ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Congestive Heart Failure [see Warnings and Precautions (5.1)] Hepatotoxicity [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Effect of TOLSURA on Other Drugs - Itraconazole and its major metabolite, hydroxy-itraconazole, are potent CYP3A4 inhibitors. Itraconazole is an inhibitor of the drug transporters ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on exposure to itraconazole during pregnancy for the approved indications. Published epidemiologic studies of women exposed to short courses of ...
-
10 OVERDOSAGEItraconazole is not removed by dialysis. In the event of accidental overdosage, supportive measures should be employed. Activated charcoal may be given if considered appropriate.
-
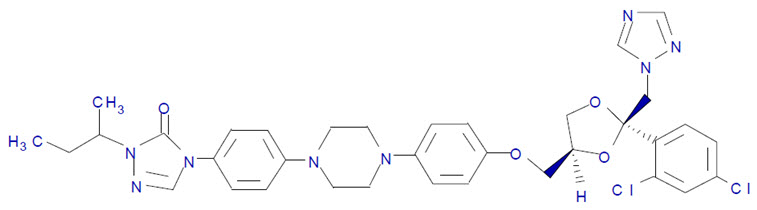
11 DESCRIPTIONTOLSURA (itraconazole capsules) is an azole antifungal drug for oral use. Itraconazole is an equal mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. It ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Itraconazole is an azole antifungal drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - General Pharmacokinetic Characteristics - The steady-state ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Itraconazole showed no evidence of carcinogenicity potential in mice treated orally for 23 months at dosage levels ...
-
14 CLINICAL STUDIESOverview of the Clinical Studies - Clinical studies in invasive mycoses listed in this section were conducted with itraconazole 100 mg capsules. Dosage for TOLSURA is different from that of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTOLSURA (itraconazole capsules) is supplied in a size 1, hard gelatin capsules with light blue cap and white body, imprinted with "i-65" in black on the cap and containing 65 mg of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Important Administration Instructions - Instruct the patients that TOLSURA: Cannot be interchanged or ...
-
SPL UNCLASSIFIED SECTIONMayne Pharma - Raleigh, NC 27609 - TOLSURA is a registered trademark of Mayne Pharma.
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 10/2024 - Patient Information - TOLSURA (tol sur ah) (itraconazole capsules) Read ...
-
PRINCIPAL DISPLAY PANEL - 65 mg Capsule Bottle LabelNDC 51862-462-60 - TOLSURA® (Itraconazole Capsules) 65 mg - Attention: Tolsura®is NOT interchangeable - on a mg per mg basis with other - formulations of itraconazole. Rx Only - 60 ...
-
INGREDIENTS AND APPEARANCEProduct Information