Label: TOLAK- fluorouracil cream
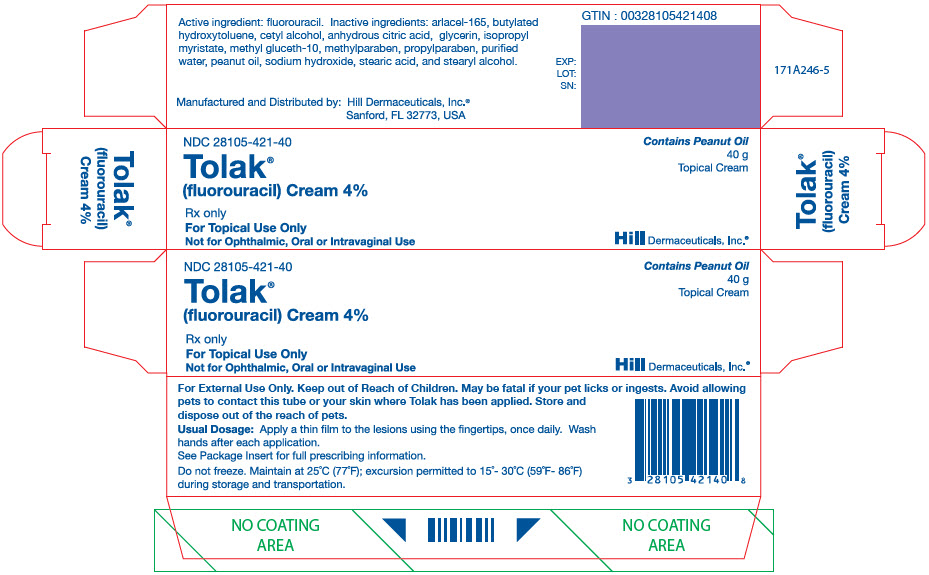
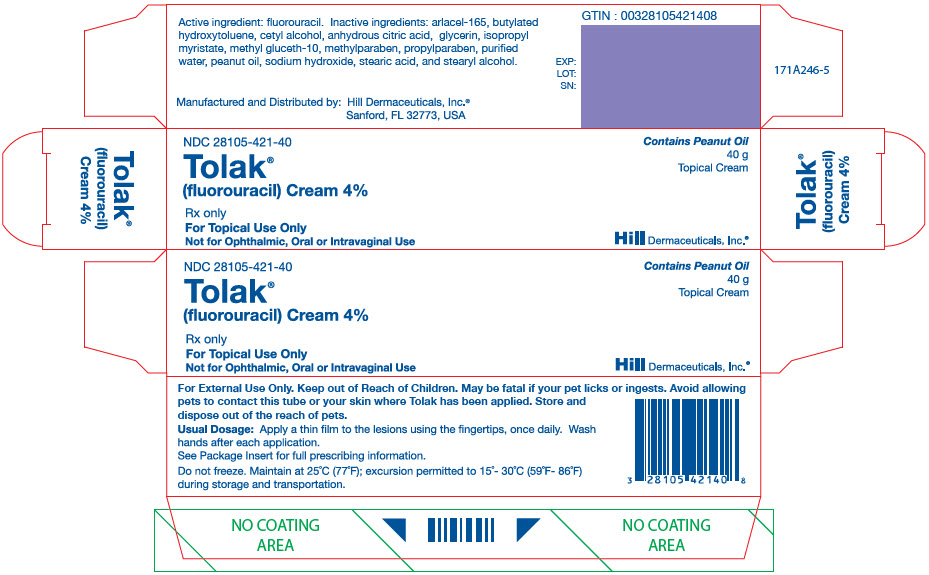
- NDC Code(s): 28105-421-40
- Packager: HILL DERMACEUTICALS, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TOLAK CREAM safely and effectively. See full prescribing information for TOLAK CREAM. TOLAK (fluorouracil) Cream, 4%, for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETolak (fluorouracil) Cream is indicated for the topical treatment of actinic keratosis lesions of the face, ears, and/or scalp.
-
2 DOSAGE AND ADMINISTRATIONPrior to application of Tolak Cream, wash, rinse, and dry the treatment areas. Apply Tolak Cream once daily in an amount sufficient to cover the lesions of the face, ears, and/or scalp with a thin ...
-
3 DOSAGE FORMS AND STRENGTHSCream: 40 mg of fluorouracil per gram (4%) of white cream in 40 gram tubes.
-
4 CONTRAINDICATIONSTolak Cream is contraindicated: During pregnancy [see Warnings and Precautions (5.5, 8.1)] In patients with dihydropyrimidine dehydrogenase (DPD) deficiency [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Application Site Adverse Reactions - Application site reactions (erythema, scaling/dryness, edema, crusting, erosions, stinging/burning, and pruritus) were observed in almost all patients ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in more detail in other sections of the labeling: Application Site Adverse Reactions [see Warnings and Precautions (5.1)] Hypersensitivity ...
-
7 DRUG INTERACTIONSSubjects using systemic steroids, immunosuppressants, and immunomodulators were generally excluded from the clinical studies of Tolak Cream, as were subjects who used retinoids, topical steroids ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects: Pregnancy Category X [see Contraindications (4.1)]. Cases of miscarriage and birth defects (including cleft lip and cleft palate) have been reported when ...
-
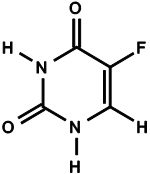
11 DESCRIPTIONTolak (fluorouracil) Cream, 4% contains 40 mg of fluorouracil per gram of white cream for topical application. It is a nucleoside metabolic inhibitor. Chemically, fluorouracil is 5-fluoro-2,4 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - There is evidence that the metabolism of fluorouracil in the anabolic pathway blocks the methylation reaction of deoxyuridylic acid to thymidylic acid. In this manner ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Adequate long-term studies in animals to evaluate carcinogenic potential of fluorouracil have not been conducted. Studies with the ...
-
14 CLINICAL STUDIESThe efficacy and safety of Tolak Cream was evaluated in two double-blind multi-center trials (Trial 1 and Trial 2) in subjects with at least 5 visible actinic keratosis lesions on the face, scalp ...
-
16 HOW SUPPLIED / STORAGE AND HANDLING 16.1 How Supplied - Tolak (fluorouracil) Cream, 4% containing 40 mg of fluorouracil per gram of white cream is available in a 40 gram tube (NDC 28105-421-40). 16.2 Storage and Handling ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Important Administration Instructions - Advise patients of the following: Tolak Cream is for external use ...
-
SPL UNCLASSIFIED SECTIONManufactured and Distributed by: Hill Dermaceuticals, Inc. Sanford, Florida 32773
-
PATIENT INFORMATION Tolak (tol lak) (fluorouracil) Cream, 4%Important: Tolak Cream is for use on skin only (topical). Do not get or apply Tolak Cream on your eyelids or in your eyes, nose, mouth, or in the vagina, because it may cause irritation and ...
-
PRINCIPAL DISPLAY PANEL - 40 g Tube CartonNDC 28105-421-40 - Tolak® (fluorouracil) Cream 4% Rx only - For Topical Use Only - Not for Ophthalmic, Oral or Intravaginal Use - Contains Peanut Oil - 40 g - Topical Cream - Hill Dermaceuticals ...
-
INGREDIENTS AND APPEARANCEProduct Information