Label: TOBRADEX ST- tobramycin / dexamethasone suspension/ drops
- NDC Code(s): 71776-035-01, 71776-035-05
- Packager: Eyevance Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONHIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use TOBRADEX ST safely and effectively. See full prescribing information for TOBRADEX ST ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETOBRADEX ST ophthalmic suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a ...
-
2 DOSAGE AND ADMINISTRATION2.1 Initiation and Continuation of Treatment - Evaluate intraocular pressure (IOP) prior to the initial prescription and renewal of the medication order - [see ...
-
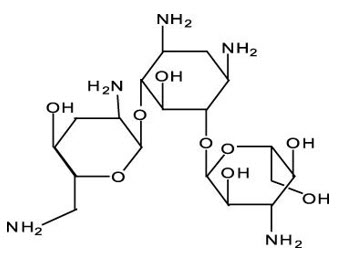
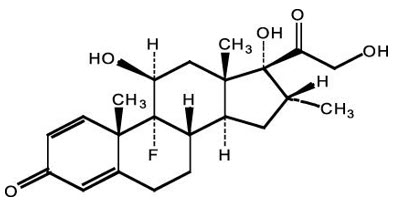
3 DOSAGE FORMS AND STRENGTHSTOBRADEX ST ophthalmic suspension 0.3%/0.05% contains 3 mg/mL tobramycin and 0.5 mg/mL dexamethasone.
-
4 CONTRAINDICATIONS4.1 Nonbacterial Etiology - TOBRADEX ST, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva, including epithelial herpes simplex ...
-
5 WARNINGS AND PRECAUTIONS5.1 Intraocular Pressure Increase - Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. If this product is used ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Corticosteroids have been shown to be teratogenic in animal studies. Ocular administration of 0.1% dexamethasone resulted in 15.6% and 32.3% incidence of fetal anomalies in 2 ...
-
11 DESCRIPTIONTOBRADEX ST (tobramycin and dexamethasone ophthalmic suspension) 0.3%/0.05% is a sterile, isotonic, white, aqueous antibiotic and steroid suspension with a pH of approximately 5.7 and an ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Dexamethasone is a potent corticoid. Corticoids suppress the inflammatory response to a variety of agents and they can delay or slow healing. Since corticoids may ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted to evaluate the carcinogenic or mutagenic potential. There are no adequate and well-controlled studies ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTOBRADEX ST is supplied as a 2.5 mL or 5 mL suspension in a 4 mL or 8 mL natural polyethylene DROP-TAINER - ® bottle with a natural polyethylene dispenser tip and a pink polypropylene overcap ...
-
17 PATIENT COUNSELING INFORMATIONStorage and Handling - Instruct the patient to store the bottle upright and away from light. Shake well before using - [see - Dosage and Administration (2.1) and ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Eyevance Pharmaceuticals, LLC - Fort Worth, TX 76102
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information