Label: TISSUEBLUE- brilliant blue g injection, solution
- NDC Code(s): 68803-722-05
- Packager: D.O.R.C. Dutch Ophthalmic Research Center (International) B.V.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONTissueBlue: These highlights do not include all the information needed to use TissueBlue 0.025% safely and effectively. See full prescribing information for TissueBlue 0.025%. Initial U.S. Approval ...
-
Table of ContentsTable of Contents
-
TissueBlue 0.025% - Indications & Usage SectionTissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is is a disclosing agent indicated to selectively stain the internal limiting membrane (ILM).
-
TissueBlue 0.025% - Dosage & Administration SectionTissueBlue 0.025% is carefully injected into the Balanced Salt Solution (BSS)-filled vitreous cavity using a blunt cannula attached to the pre-filled syringe, without allowing the cannula to ...
-
TissueBlue 0.025% - Dosage forms & Strengths sectionTissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is a clear, bright blue, single-dose ophthalmic solution supplied in 2.25 mL syringes pre-filled to a volume of 0.5 mL.
-
TissueBlue 0.025% - Contraindications sectionNone
-
TissueBlue 0.025% - Warnings and Precautions sectionExcessive Staining - Excess TissueBlue 0.025% should be removed from the eye immediately after staining. Use of the Syringe - Make sure the plunger moves smoothly ...
-
TissueBlue 0.025% - Adverse Reactions sectionAdverse reactions that have been reported in procedures that included the use of Brilliant Blue G Ophthalmic Solution have often been associated with the surgical procedure. These complications ...
-
TissueBlue 0.025% - Use in specific populations sectionTissueBlue 0.025% - Pregnancy section - Risk Summary - There are no available data on the use of TissueBlue 0.025% in pregnant women to inform a drug associated risk. Systemic ...
-
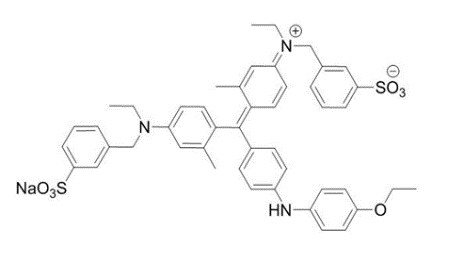
TissueBlue 0.025% - Description sectionTissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is a sterile solution of BBG (a dye). Each mL of TissueBlue 0.025% contains BBG 0.25 mg, Polyethylene Glycol 40mg and Buffered Sodium ...
-
TissueBlue 0.025% - Clinical Pharmacology sectionTissueBlue 0.025% - Mechanism of action section - Brilliant Blue G has been shown to selectively stain the ILM, but not the epiretinal membrane nor the retina, making it easier to visualize the ...
-
TissueBlue 0.025% - Nonclinical toxicology sectionTissueBlue 0.025% - Nonclinical toxicology section - Studies to evaluate the potential for carcinogenicity or impairment of fertility of TissueBlue 0.025% have not been conducted ...
-
TissueBlue 0.025% - How supplied sectionTissueBlue (Brilliant Blue G Ophthalmic Solution), 0.025% is supplied as 0.5 mL of Brilliant Blue G Ophthalmic Solution, 0.025% in a sterile, single-dose Luer Lok, 2.25 mL glass syringe, with a ...
-
TissueBlue 0.025% - Storage and Handling sectionTissueBlue 0.025% should be stored at 15°C to 25°C (59°F to 77°F). Protect from light, frost and moisture.
-
SPL UNCLASSIFIED SECTIONRx Only - Distributed by: Dutch Ophthalmic, USA - 10 Continental Drive, Bldg 1 - Exeter, NH 03833, USA - Phone: 800-75-DUTCH or 603-778-6929 ...
-
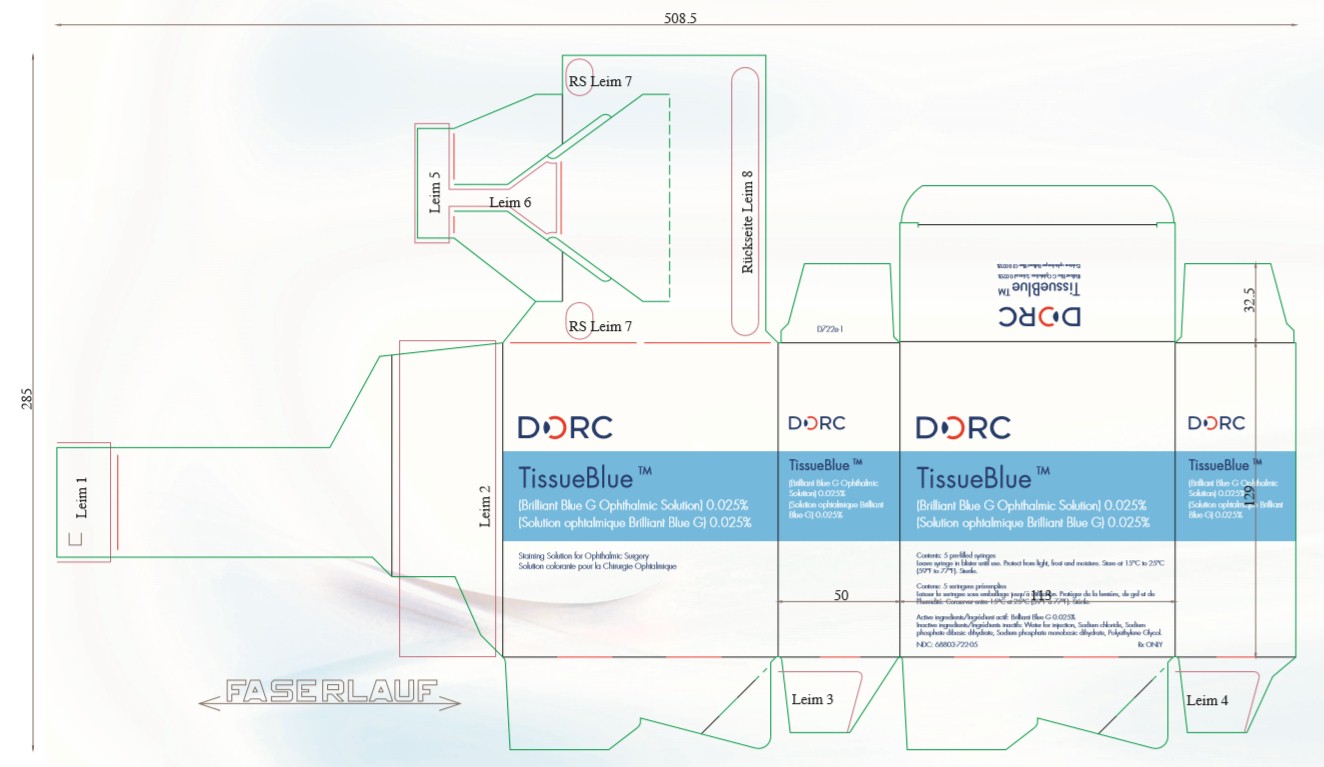
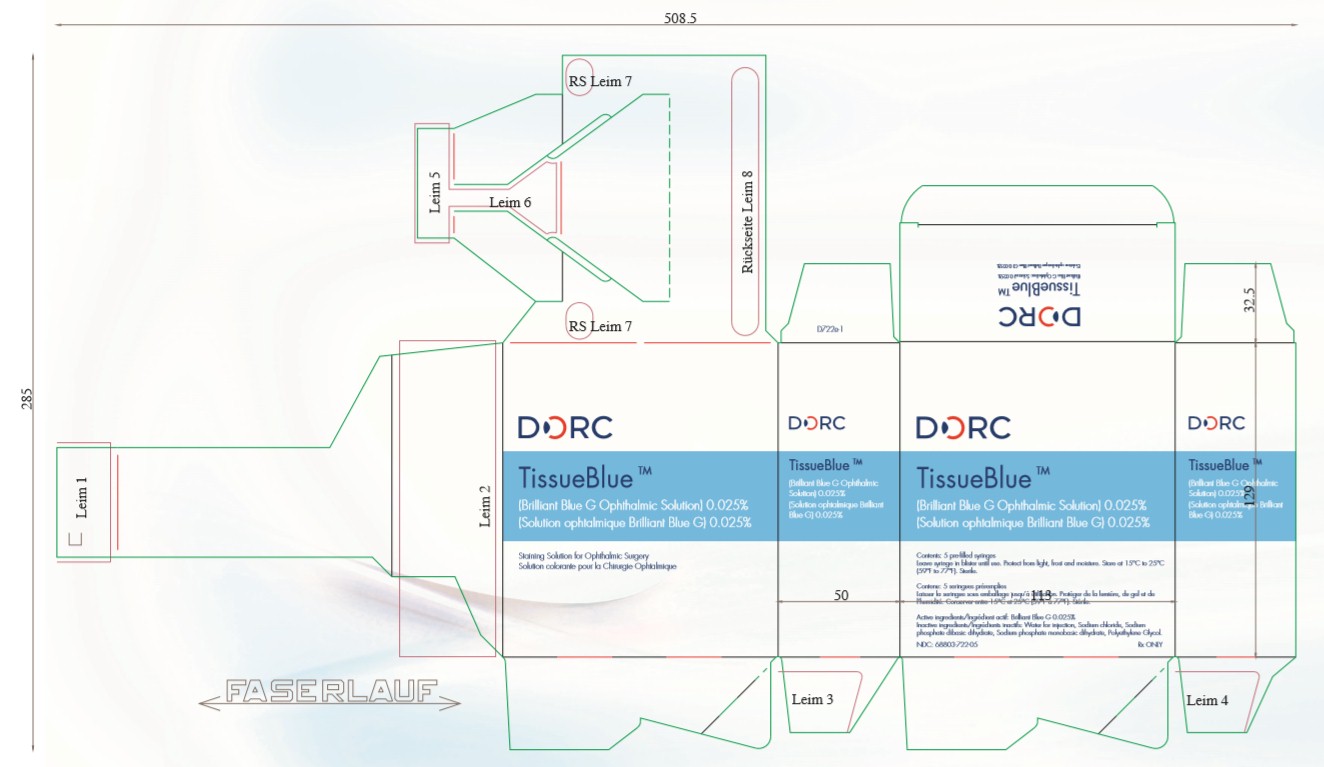
Package Label - 0.5 mLTissueBlue - (Brilliant Blue G Ophthalmic Solution) 0.025% Staining Solution for Ophthalmic Surgery - Protect from light, frost and moisture. Store at 15°C to 25°C (59°F to 77°F). Sterile. Active ...
-
INGREDIENTS AND APPEARANCEProduct Information