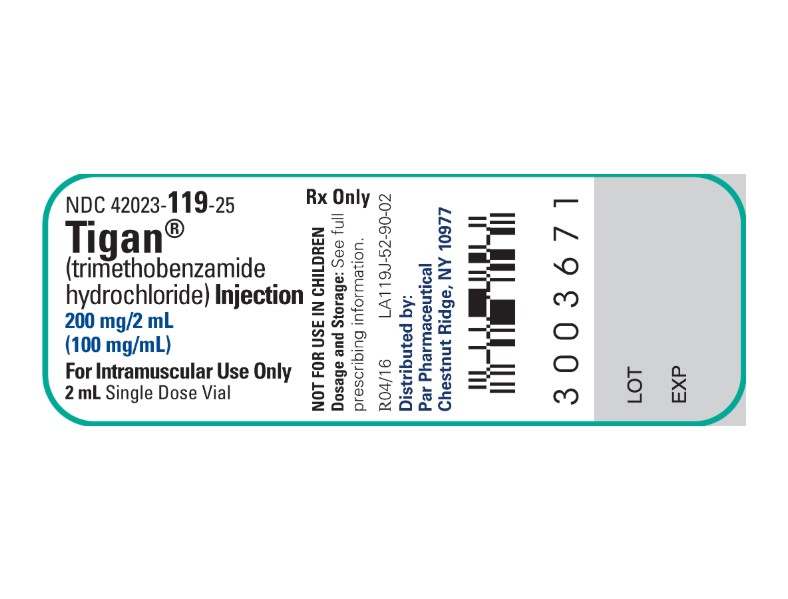
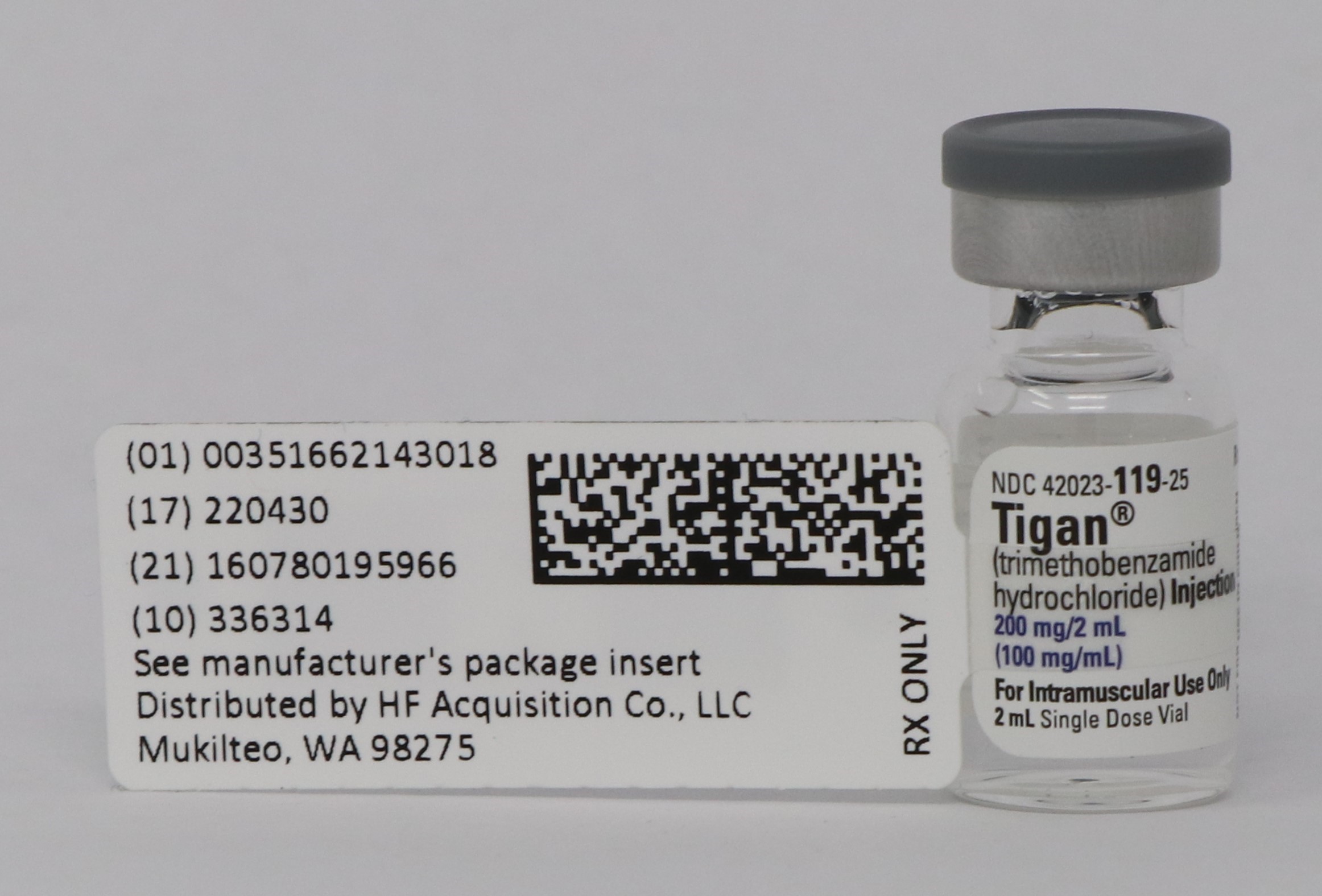
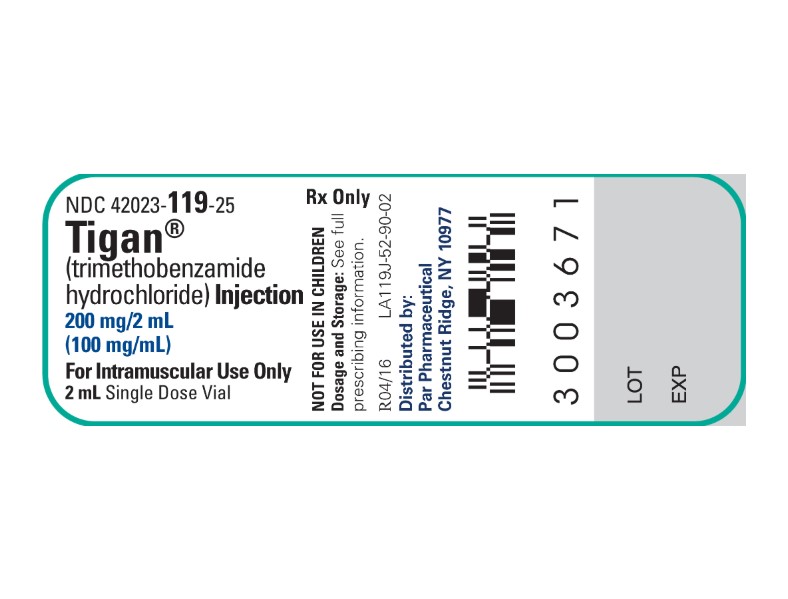
Label: TIGAN(R)- TRIMETHOBENZAMIDE HYDROCHLORIDE injection
- NDC Code(s): 51662-1430-1, 51662-1430-2, 51662-1430-3
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 42023-119
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIEDInjectable - For Intramuscular Use Only - Not for Use in Pediatric Patients
-
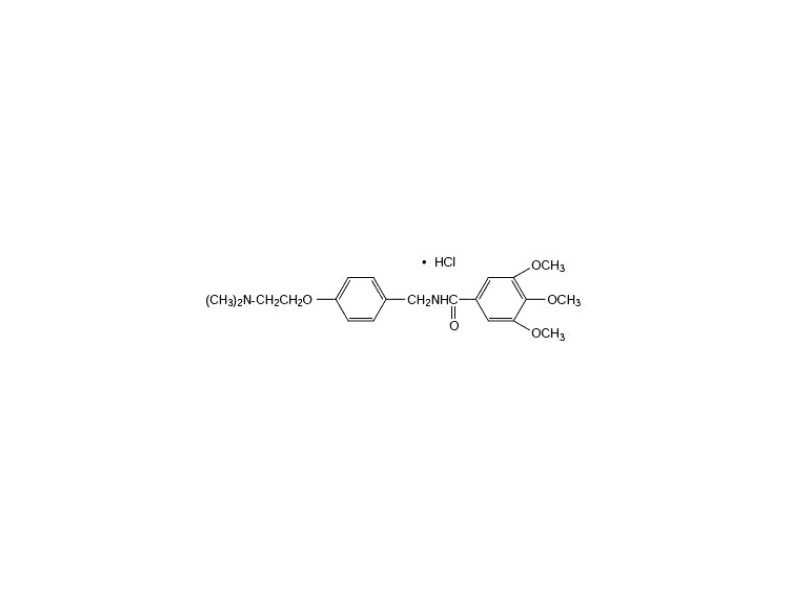
DESCRIPTIONChemically, trimethobenzamide hydrochloride (HCl) is N-[p-[2-(dimethylamino)ethoxy]benzyl]-3,4,5-trimethoxybenzamide monohydrochloride. It has a molecular weight of 424.93 and the following ...
-
CLINICAL PHARMACOLOGYMechanism of Action - The mechanism of action of Tigan® as determined in animals is obscure, but may involve the chemoreceptor trigger zone (CTZ), an area in the medulla oblongata through which ...
-
INDICATIONSTigan® is indicated for the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis.
-
CONTRAINDICATIONSThe injectable form of Tigan® is contraindicated in pediatric patients and in patients with known hypersensitivity to trimethobenzamide.
-
WARNINGSTigan® may produce drowsiness. Patients should not operate motor vehicles or other dangerous machinery until their individual responses have been determined. Usage in Pregnancy - Trimethobenzamide ...
-
PRECAUTIONSDuring the course of acute febrile illness, encephalitides, gastroenteritis, dehydration and electrolyte imbalance, especially in children and the elderly or debilitated, CNS reactions such as ...
-
ADVERSE REACTIONSThere have been reports of hypersensitivity reactions and Parkinson-like symptoms. There have been instances of hypotension reported following parenteral administration to surgical patients. There ...
-
DOSAGE & ADMINISTRATION(See - WARNINGS and - PRECAUTIONS.) Dosage should be adjusted according to the indication for therapy, severity of symptoms and the response of the patient. Geriatric Patients - Dose adjustment ...
-
STORAGEStore between 20° to 25°C (68° to 77°F). (See USP Controlled Room Temperature.)
-
HOW SUPPLIEDTIGAN(R) (TRIMETHOBENZAMIDE HYDROCHLORIDE) INJECTION is supplied in the following dosage forms. NDC 51662-1430-1 - TIGAN(R) (TRIMETHOBENZAMIDE HYDROCHLORIDE) INJECTION 200mg/2mL (100mg/mL ...
-
SPL UNCLASSIFIEDRx Only - Distributed by: Par Pharmaceutical - Chestnut Ridge, NY 10977 - R04/16 - OS118J-01-90-02 - 3000358G
-
PRINCIPAL DISPLAY PANEL - VIAL LABEL
-
PRINCIPAL DISPLAY PANEL - SERIALIZED LABELING
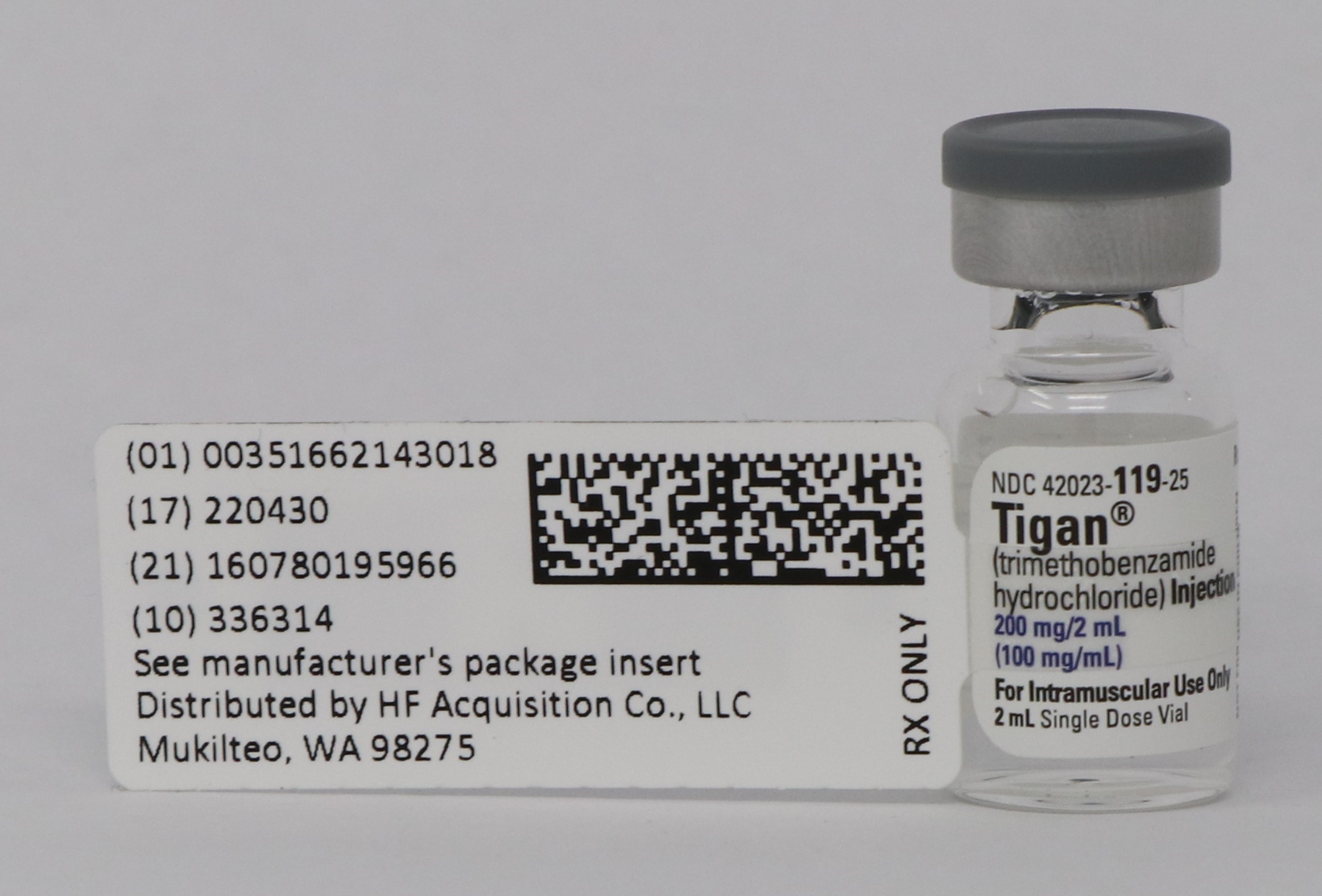
-
PRINCIPAL DISPLAY PANEL NDC 51662-1430-2 POUCH LABELINGNDC 51662-1430-2 POUCH LABELING - VIAL LABELING
-
PRINCIPAL DISPLAY PANEL - NDC 51662-1430-3 CASE LABELINGNDC 51662-1430-3 CASE LABELING - SERIALIZED CASE RFID LABELING
-
INGREDIENTS AND APPEARANCEProduct Information