Label: TEPEZZA- teprotumumab injection, powder, lyophilized, for solution
- NDC Code(s): 75987-130-15
- Packager: Horizon Therapeutics USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TEPEZZA safely and effectively. See full prescribing information for TEPEZZA. TEPEZZA (teprotumumab-trbw) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETEPEZZA is indicated for the treatment of Thyroid Eye Disease regardless of Thyroid Eye Disease activity or duration.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - The recommended dose of TEPEZZA is an intravenous infusion of 10 mg/kg for the initial dose followed by an intravenous infusion of 20 mg/kg every three weeks for 7 ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection (intravenous infusion): 500 mg of teprotumumab as a white to off-white lyophilized powder in a single-dose vial for reconstitution and dilution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Infusion Reactions - TEPEZZA may cause infusion reactions. Infusion reactions have been reported in approximately 4% of patients treated with TEPEZZA. Signs and symptoms of ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Infusion Reactions [see Warnings and Precautions (5.1)] Exacerbation of Preexisting Inflammatory ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals and its mechanism of action inhibiting insulin-like growth factor 1 receptor (IGF-1R), TEPEZZA may cause fetal harm when ...
-
10 OVERDOSAGENo information is available for patients who have received an overdosage.
-
11 DESCRIPTIONTeprotumumab-trbw, an insulin-like growth factor-1 receptor inhibitor (IGF-1R), is a fully human IgG1 monoclonal antibody produced in Chinese hamster ovary (CHO-DG44) cells. It has a molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Teprotumumab-trbw's mechanism of action in patients with Thyroid Eye Disease has not been fully characterized. Teprotumumab-trbw binds to IGF-1R and blocks its ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of TEPEZZA has not been evaluated in long-term animal studies. Mutagenesis - The ...
-
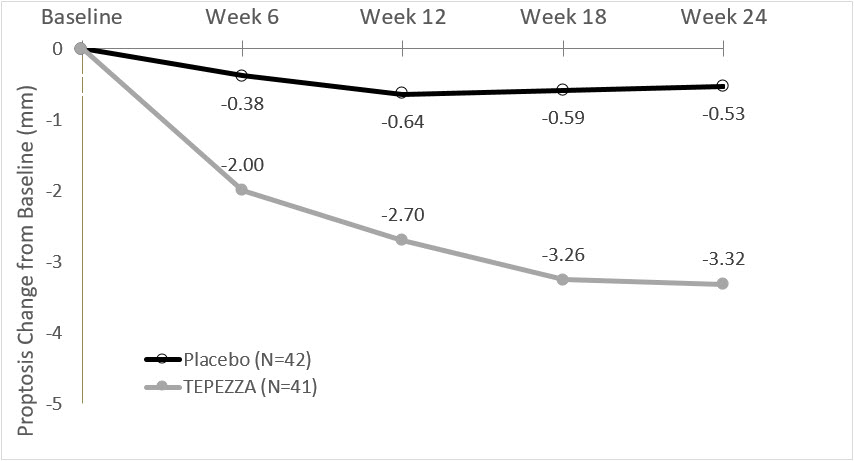
14 CLINICAL STUDIESTEPEZZA was evaluated in 2 randomized, double-masked, placebo-controlled studies in 171 patients with Thyroid Eye Disease: Study 1 (NCT01868997) and Study 2 (NCT03298867). Patients were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTEPEZZA (teprotumumab-trbw) for injection is a sterile, preservative-free, white to off-white lyophilized powder available as follows: Carton containing one 500 mg single-dose vialNDC ...
-
17 PATIENT COUNSELING INFORMATIONEmbryo-Fetal Toxicity - Advise females of reproductive potential that TEPEZZA can cause harm to a fetus and to inform their healthcare provider of a known or suspected pregnancy. Educate and ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Horizon Therapeutics Ireland DAC - Dublin, Ireland - U.S. License No. 2022 - Patent: http://pat.amgen.com/tepezza/ © 2025 Amgen Inc. All rights reserved - 1-800-772-6436 - 1XXXXXX-V6
-
PRINCIPAL DISPLAY PANEL - 500 mg Vial CartonNDC-75987-130-15 - TEPEZZA® (teprotumumab-trbw) for Injection - 500 mg/vial - For Intravenous Infusion Only - Reconstitute and Further Dilute Prior to Use - Single-dose vial. Discard unused portion. Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information