Label: TECHNESCAN MAG3- technescan tc 99m mertiatide injection, powder, lyophilized, for solution
- NDC Code(s): 69945-096-20
- Packager: Curium US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
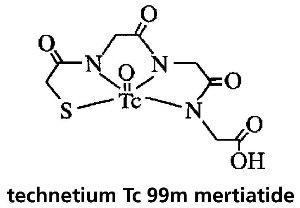
DESCRIPTIONTechnescan MAG3™ is a kit for the preparation of technetium Tc 99m mertiatide, a diagnostic radiopharmaceutical. It is supplied as a sterile, nonpyrogenic, lyophilized powder. Each vial contains ...
-
PHYSICAL CHARACTERISTICSTechnetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.1The principal photon that is useful for detection and imaging is listed in Table 1. Table 1. Principal ...
-
CLINICAL PHARMACOLOGYFollowing intravenous injection of technetium Tc 99m mertiatide, the appearance, concentration, and excretion of the tracer in the kidney can be monitored to assess renal function. Although ...
-
INDICATIONS AND USAGETechnetium Tc 99m mertiatide is a renal imaging agent for use in the diagnosis of congenital and acquired abnormalities, renal failure, urinary tract obstruction, and calculi in adults and ...
-
CONTRAINDICATIONSNone known.
-
WARNINGSNone known.
-
PRECAUTIONSGeneral - The contents of this kit are not radioactive. However, after sodium pertechnetate Tc 99m is added, adequate shielding of the final preparation must be maintained. Contents of the ...
-
ADVERSE REACTIONSThe following adverse reactions have been reported: nausea, vomiting, wheezing, dyspnea, itching, rash, tachycardia, hypertension, shaking chills, fever, and seizure.
-
DOSAGE AND ADMINISTRATIONThe suggested dose range employed in the average adult patient (70 kg) for renal function and imaging studies is 185 MBq (5 mCi) to 370 MBq (10 mCi). In pediatric patients the recommended dose ...
-
RADIATION DOSIMETRYThe estimated absorbed radiation doses from an intravenous administration of technetium Tc 99m mertiatide are presented in Table 4.
-
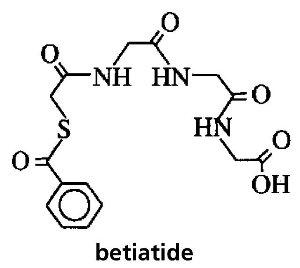
HOW SUPPLIEDCatalog Number 096. Technescan MAG3 is supplied as a lyophilized powder packaged in vials. Each reaction vial contains 1 mg betiatide, 0.05 mg (minimum) stannous chloride dihydrate (SnCl2•2H2O) ...
-
STORAGETechnescan MAG3 should be stored at controlled room temperature 20° to 25°C (68° to 77°F) and protected from light until use. The reconstituted vial should be stored at room temperature (15° to ...
-
INSTRUCTIONS FOR THE PREPARATION OF TECHNETIUM Tc 99m MERTIATIDENote: Read complete directions thoroughly before starting preparation procedure. Procedural Precautions and Notes - Solutions of sodium pertechnetate Tc 99m which contain oxidizing agents ...
-
RECOMMENDED METHOD FOR DETERMINATION OF RADIOCHEMICAL PURITY OF Technescan MAG3™Required Materials: Waters Sep-Pak™ C18 Cartridges, Part #51910, 200 proof ethanol - 0.9% Sodium Chloride Injection, USP - 0.001N hydrochloric acid* 1:1 ethanol/saline solution** Disposable ...
-
PRINCIPAL PANEL DISPLAY - A096V0Technescan MAG3™ Kit for the Preparation of Technetium Tc 99m Mertiatide Diagnostic - Vial contains: 1 mg Betiatide - 0.05 mg (minimum) Stannous Chloride Dihydrate (SnCl2 ● 2H2O) 40 mg Sodium ...
-
INGREDIENTS AND APPEARANCEProduct Information